Abstract
Background
Adequate antiretroviral exposure is crucial for virological suppression. We assessed the relationship between atazanavir hair levels with self-reported adherence, virological outcomes, and the effect of a home-based adherence intervention in HIV-infected adolescents failing 2nd-line antiretroviral treatment in Zimbabwe.
Methods
HIV-infected adolescents on atazanavir/ritonavir-based 2nd-line treatment for ≥6 months with viral load (VL) >1,000 copies/ml were randomised to either standard care (control) or standard care plus modified directly administered antiretroviral therapy (mDAART) (intervention). Questionnaires were administered; VL and hair samples were collected at baseline and after 90 days in each group. Viral suppression was defined as <1,000 copies/ml after follow-up.
Results
Fifty adolescents (10–18 years) were enrolled; 23(46%) were randomized to intervention and 27(54%) to control. Atazanavir hair concentration <2.35ng/mg (lower inter-quartile range for those with virological suppression) defined a cut-off below which most participants experienced virological failure. Male sex (p=0.03), virological suppression at follow-up (p=0.013), greater reduction in VL (p=0.006) and change in average self-reported adherence over the previous month (p=0.031) were associated with adequate (>2.35ng/mg) hair concentrations. Participants with virological failure were more likely to have sub-optimal atazanavir hair concentrations (RR=7.2, 95% CI=1–51, p=0.049). There were no differences in atazanavir hair concentration between the arms after follow-up.
Conclusion
A threshold of atazanavir concentrations in hair (2.35ng/mg), above which virological suppression was likely, was defined for adolescents failing 2nd line atazanavir/ritonavir-based ART in Zimbabwe. Male sex and better self-reported adherence were associated with adequate atazanavir hair concentrations. Antiretroviral hair concentrations may serve as a useful clinical tool among adolescents.
Keywords: Adolescents, virological failure, atazanavir hair concentrations
Introduction
Sustaining optimal antiretroviral (ARV) exposure in HIV-infected adolescents is challenging. Treatment adherence may be compromised by the transition to adulthood while coming to terms with their HIV status for the first time, leading to anger, denial and rebellion at a time when there is desire for peer conformity1. Perinatally-infected adolescents often have long treatment histories and may be multi-drug experienced, leading to treatment fatigue, inadequate adherence, accumulation of drug resistant strains and treatment failure2. Moreover, when to switch from weight-based to age-based dosing remains unclear. Perinatally-infected adolescents can demonstrate low body mass index-for-age (BMI-for-age)3, which can lead to toxicity if ARVs are dosed by age or inadequate exposure if dosed by weight4. Therefore, defining metrics to assess ARV adherence and exposure is critical for monitoring adolescents living with HIV infection.
Accurate measurement of drug exposure is most crucial in the setting of treatment failure, especially when self-reported adherence is claimed to be adequate. Measurement of drug levels in hair integrates, in one assay, both biology (pharmacokinetics) and behavior (adherence) in the preceding weeks to months5. Hair grows at about 1cm/month, incorporating drugs from the circulation as it grows6. Sub-therapeutic ARV drug levels in hair could be a result of sub-optimal adherence, reduced absorption or increased clearance. Supra-therapeutic levels can result in toxicity, leading to non-adherence.
The utility of hair concentrations as a metric of adherence and exposure in HIV-infected adults has been explored,5,8 but the study of this measure in adolescents is more limited7. This study sought to assess the relationship between atazanavir hair concentration and virological outcomes, as well as self-reported adherence, among HIV-infected adolescents with virological failure on atazanavir/ritonavir-based 2nd-line treatment in Harare, Zimbabwe. We also sought to determine if a home-based adherence intervention increased atazanavir concentrations in hair in the same cohort.
Methods
Study procedures were described earlier9. Briefly, this was a randomised, controlled trial conducted among 50 HIV-positive adolescents at Harare Hospital, Zimbabwe, with virological failure (defined by HIV viral load ≥1,000 copies/ml) for ≥6 months on atazanavir/ritonavir-based 2nd-line treatment. The intervention, modified directly administered antiretroviral therapy (mDAART), consisted of structured home visits during the week and SMS text messages during weekends plus standard of care over 90 days9. The control arm received standard of care alone. At baseline and follow-up, questionnaires were administered using validated instruments, and hair and viral load samples collected10;11.
Hair was collected according to a standard protocol and stored at room temperature until analysis12. Samples were shipped to the University of California, San Francisco (UCSF) Hair Analytical Laboratory (HAL) where hair assays were developed, validated, peer reviewed and approved by the Division of AIDS Clinical Pharmacology and Quality Assurance (CPQA)13. Participants without baseline and follow-up hair samples were excluded from this analysis.
Atazanavir hair concentrations were measured using liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS), with an assay range of 0.05–20ng/mg. Of note, the UCSF HAL has previously shown that atazanavir hair levels do not vary significantly by race or ethnicity14.
The study was registered with the Pan African Clinical Trial Registry (PACTR201502001028169) and NIH Clinical Trials.gov (NCT02689895), and approved by Harare hospital institutional review board, Joint Research Ethics Committee (JREC/51/14), Biomedical and Research Training Institute, Medical Research Council of Zimbabwe (MRCZ/A/1840), Research Council of Zimbabwe (Ref: 02810) and UCSF Committee on Human Research (CHR #11-07442).
Statistical considerations
Data was entered into Research Electronic Data Capture (REDCap), and analysed in Stata 1415;16. Chi-square (and Fisher’s exact test where appropriate) and Student’s t-tests were used to determine associations between atazanavir hair concentrations and virological outcomes at follow-up, as well as with study group and self-reported adherence. P-values (two-sided) were considered statistically significant if ≤0.05. Treatment outcome assessed was viral load suppression to <1,000 copies/ml.
Factors demonstrating p-values <0.25 in bivariate analysis and possible confounders were included in stepwise multivariate logistic regression models assessing the relationship between the mDAART intervention and virological suppression. These factors included: age; gender; World Health Organisation (WHO) clinical stage at ART initiation; latest CD4 cell count; total time on ART; viral load at follow-up; self-reported adherence (assessed by visual analogue scale and AIDS Clinical Trials Group (ACTG) adherence follow-up questionnaire (QLO702)), atazanavir hair concentrations and BMI-for-age17.
Sample size determination was carried out post-hoc on the basis of comparing study arms and follow-up viral load (suppressed and unsuppressed) relative to log-transformed follow-up atazanavir hair concentrations using the “power twomeans” function in STATA. The mean log transformed hair concentration was 1.5 (SD=0.6) and 1.1 (SD=0.4) for the control and intervention groups respectively. Therefore, a sample size of 42 gives 80% power. The mean log transformed atazanavir hair concentration was 1.6 (SD=0.7) and 1.0 (SD=0.5) for suppressed and unsuppressed groups respectively; therefore, a sample size of 42 gives 83% power.
Results
Participant demographics and acceptability of hair collection
All 50 adolescents, median age 16 years, 27 females and 23 males receiving atazanavir/ritonavir had mean viral load log10 4.8 copies/ml at baseline9. Twenty-three were randomised to intervention and 27 were randomised to control arms. Two (4%) participants declined to have hair collected both at baseline and during follow-up because they did not want their hairstyles disrupted. Four (8%) and 6(12%) cut their hair too short before baseline and follow-up visits, respectively, to allow for collection, leading to an overall collection rate of 86%.
Effect of mDAART intervention
Study arms were well matched (supplement table 1). At baseline, mean atazanavir hair concentration was 1.4ng/mg. Forty percent reported average adherence<80% over the previous 1 month, 30% were underweight while 15% were overweight. After follow-up, the mDAART intervention resulted in significantly lower viral load (p=0.048), greater reduction in viral load (p=0.031), higher average self-reported adherence over the previous month (p=0.05), higher following of the dosing schedule by self-report in the previous 4 days (p<0.001) and a trend towards increased virological suppression rates to <1,000 copies/ml (p=0.105) (supplement table 2). Atazanavir hair concentrations did not differ significantly in the 2 arms. In multivariate analysis, participants in mDAART were more likely to report closely following dosing schedule in the past 4 days (RR=10, 95% CI=1.9–52, p=0.007) (supplement table 3).
Factors associated with virological suppression across both study arms
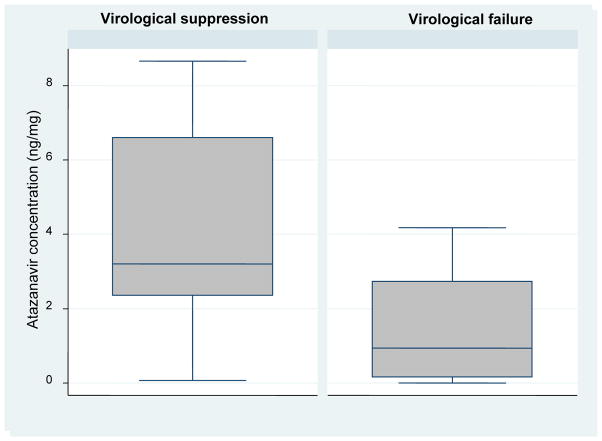
The median and inter-quartile range (IQR) of atazanavir hair concentrations among those with virological suppression and failure were (n=18; median hair level 3.21, IQR 2.35–6.61ng/mg) and (n=24; median hair level 0.94, IQR 0.16–2.73ng/mg) respectively. The value of 2.35ng/mg (lower IQR for those with virological suppression) defined a cut-off below which most participants experienced virological failure (figure 1 and supplement table 4).
Figure 1.
Box and whisker plots showing atazanavir concentration in hair by virological outcome after follow-up (p<0.001)
The following characteristics were associated with adequate (>2.35ng/mg) atazanavir hair concentrations: male sex (p=0.03); virological suppression after follow-up (p=0.013); greater reduction in viral load (p=0.006) and change in average self-reported adherence over the previous 1 month at baseline and at follow-up (p=0.031) (supplement table 5).
In multivariate analysis, sub-optimal (≤2.35ng/mg) atazanavir hair concentrations were strongly associated with virological failure (RR=7.2, 95% CI=1–51, p=0.049) (table 1).
Table 1.
Multivariate logistic regression to determine factors associated with virological failure
| Variable | Relative risk (95% confidence interval) | p-value |
|---|---|---|
|
| ||
| Hair atazanavir concentration at follow-up (ng/mg): | ||
| ≤2.35 | 7.2(1–51) | 0.049 |
|
| ||
| Study arm: | ||
| mDAART | 0.26(0.04–1.62) | 0.148 |
|
| ||
| WHO clinical stage at ART initiation: | ||
| 3–4 | 1.5(0.3–7.6) | 0.613 |
|
| ||
| Self-reported closely following dosing schedule in past 4 days at follow-up: | ||
| Yes | 1.05(0.19–6) | 0.953 |
|
| ||
| Gender: | ||
| Male | 1.6(0.26–10.1) | 0.598 |
|
| ||
| Latest CD4 cell count (cells/mm3): | ||
| 200–350 | 1.3(0.2–8.8) | 0.770 |
| >350 | 0.6(0.09–4) | 0.591 |
|
| ||
| Average self-reported adherence, VAS, at follow-up: | ||
| 80–94% | 0.28(0.05–1.7) | 0.162 |
| <80% | 0.61(0.05–6.8) | 0.689 |
mDAART- modified directly administered antiretroviral therapy; WHO- World Health Organisation; ART- antiretroviral therapy; VAS- visual analogue scale
Discussion
In this cohort of adolescents living with HIV infection on 2nd line ART, atazanavir concentrations in small hair samples were the strongest independent predictor of virological suppression. Although other studies have shown this association in adults, our study is the first to explore this association in adolescents with confirmed virological 2nd line treatment failure5. Moreover, we have defined in this study a threshold of atazanavir hair concentrations (2.35ng/mg) above which most participants experienced virological suppression and below which most participants experienced virological failure. Defining such a cut-off allows hair levels to serve as a useful tool in the clinical setting.
Adolescents are vulnerable to inadequate adherence. Early identification of sub-optimal atazanavir hair concentrations and timely interventions in HIV-infected adolescents may ultimately reduce HIV-related morbidity and mortality, and curb the spread of HIV infection and drug resistant HIV strains in this vulnerable group. Sub-optimal drug levels could trigger intensive adherence interventions before advanced treatment failure sets in, and before evolution of drug resistance occurs. Measurement of antiretroviral drug levels may reduce the need for expensive ARV drug resistance testing, allowing this to be reserved for patients who fail treatment with adequate drug levels in resource-limited settings8. Where viral load measurement is prohibitively expensive, drug levels in hair, if performed economically, can serve as a surrogate for viral loads.
The acceptance rate for hair collection in our Harare-based adolescent cohort was high and similar to rates reported in Kenya6. This finding is encouraging, especially for resource-limited settings where collection of biological samples for research can be challenging due to participant distrust or superstition18. Hair collection is simple and non-invasive compared to venipuncture, which may increase acceptability and feasibility in children and adolescents who are afraid of needles.
The mDAART intervention was not correlated with atazanavir hair concentrations, but there was a trend towards higher virological suppression rates in those receiving the intervention. Moreover, mDAART participants had increased self-reported adherence after follow-up. Hair concentrations, which reflect accumulation of drug levels from the systemic circulation, may have lagged behind initial increases in plasma drug levels. Studies with longer follow-up in the setting of an ongoing intervention are indicated.
After follow-up, some participants had sub-optimal atazanavir hair concentrations while being virologically suppressed, while some virologically failing participants had adequate atazanavir hair concentrations. These findings could reflect a lag in accumulation of atazanavir in hair compared to plasma concentrations and a lag in viral load suppression as antiretroviral exposure increases, respectively. Failure despite adequate hair levels could also occur in the setting of virological resistance, necessitating genotype. Studies of longer duration could provide clarity. Finally, despite statistical association, there was poor correlation between self-reported adherence and atazanavir hair concentrations. The most likely explanation for this discrepancy is that self-reported adherence is often overestimated due to social desirability.19
In our study, males were more likely to have adequate atazanavir hair concentrations (defined by hair level of 2.35ng/mg), consistent with other studies that have found that adherence in adolescent females is generally lower compared to males4. Girls enter adolescence earlier than boys, and hence may be more susceptible to peer-conformity/pressure and earlier lapses in adherence. Moreover, girls have earlier sexual debut than boys, with contraception and early child-bearing potentially reducing ART exposure20.
BMI-for-age was not associated with atazanavir hair concentrations. Previous studies report contradictory findings4. However, these studies were conducted in adult patients, and plasma atazanavir concentrations were measured. This finding needs to be evaluated further in adolescents and with atazanavir hair concentrations.
Our study had a few limitations. Frequent home visits, higher at the beginning of the intervention decreased as the intervention progressed to reduce intrusiveness and maintain acceptability. Participants could have reduced their adherence as the visits decreased, resulting in sub-optimal atazanavir hair levels and virological failure. Plasma drug levels were not measured. However, plasma concentrations only distinguish participants who adhered to treatment as their review days approached (“white coat adherence”) while hair concentrations assess adherence over the past weeks to months. In addition, recruitment was done at one centre, limiting generalizability.
Conclusion
Measurement of antiretroviral drug levels in hair allows for measurement of both drug exposure and adherence, and predicts virological treatment outcome in multiple cohorts, including our sample of HIV-infected adolescents in Harare. We were able to define a threshold of atazanavir concentrations in hair (2.35ng/mg), above which there were high rates of virological suppression among adolescents on 2nd line atazanavir/ritonavir-based ART. As in previous studies in adults, atazanavir hair levels were the strongest independent predictor of virological suppression in our cohort. The mDAART intervention did not increase atazanavir hair levels in our small study with a limited follow-up period but was associated with a trend towards increased virological suppression rates. Hair levels may be a useful tool in resource-constrained settings with limited future treatment options to measure ARV adherence and should be further explored among HIV-infected adolescents.
Supplementary Material
Acknowledgments
We would like to thank Dr. Leslie Z. Benet, Karen Kuncze and Nhi Phung from the UCSF HAL for work on hair assays; Dr Rashida Ferrand and Dr Hildah Mujuru for providing guidance; Child Protection Society for providing trained community workers, and Noleen Chifamba, the research nurse.
Sources of funding: University of Zimbabwe Staff Development Fellowship (SDF), Fogarty HIV Implementation Science Research Training Program (FHISRTP) (1D43TW009539-03), Fogarty International Clinical, Operational and Health Services Research and Training Award (ICOHRTA) (2U2RTW007367-01) and National Institute for Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH) (2R01 AI098472, PI- Monica Gandhi).
Footnotes
Preliminary findings: Preliminary results were presented as a poster at the International AIDS Conference (IAS) in Durban, South Africa, 18–22 July 2016.
Conflicts of interest: All authors declare no conflict of interest. The contents of this publication are solely the responsibility of the authors, and do not represent the views of the funders.
References
- 1.Bakanda C, Birungi J, Mwesigwa R, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: findings from a nationally representative cohort in Uganda. PLoS One. 2011;6:e19261. doi: 10.1371/journal.pone.0019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claborn KR, Meier E, Miller MB, et al. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2015;20:255–265. doi: 10.1080/13548506.2014.945601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–639. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18579. doi: 10.7448/IAS.16.1.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52:1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey MD, Salmen CR, Tessler RA, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66:311–315. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasitsuebsai W, Kerr SJ, Truong KH, et al. Using lopinavir concentrations in hair samples to assess treatment outcomes on second-line regimens among Asian children. AIDS Res Hum Retroviruses. 2015;31:1009–1014. doi: 10.1089/aid.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Zyl GU, van Mens TE, McIlleron H, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56:333–339. doi: 10.1097/QAI.0b013e31820dc0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawana TD, Katzenstein D, Nathoo K, et al. Evaluating an enhanced adherence intervention among HIV positive adolescents failing atazanavir/ritonavir-based second line antiretroviral treatment at a public health clinic. J AIDS HIV Res. 2017;9:17–30. doi: 10.5897/JAHR2016.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 12.The Women’s Interagency HIV Study. WIHS manual of operations. Section 24. Hair collection protocol. 2013 [Google Scholar]
- 13.DiFrancesco R, Tooley K, Rosenkranz S, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93:479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Gandhi M, Greenblatt RM, et al. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture- a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistical software: Release 14.0. College Station, TX: Stata Corporation; 2001. [Google Scholar]
- 17.World Health Organisation Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr. 2007;95:76–85. [Google Scholar]
- 18.Coetzee B, Kagee A, Tomlinson M, et al. Reactions, beliefs and concerns associated with providing hair specimens for medical research among a South African sample: a qualitative approach. Future Virol. 2012;7:1135–1142. doi: 10.2217/fvl.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43:S79–S87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyamukapa CA, Gregson S, Lopman B, et al. HIV-associated orphanhood and children’s psychosocial distress: theoretical framework tested with data from Zimbabwe. Am J Public Health. 2008;98:133–141. doi: 10.2105/AJPH.2007.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.