Introduction
Cancer survivorship is a priority area of focus for the National Cancer Institute (NCI). There were an estimated 15.5 million survivors alive at the beginning of 2016 [17]. The contribution of population aging to an increased incidence of cancer coupled with improved treatment efficacy (especially in the early stages of disease) means this number will continue to rise. Many survivors are at increased risk of cancer recurrence, developing secondary cancers and comorbidities such as cardiovascular disease [14] and diabetes [5], which may impact their long-term quality of life (QOL). Endometrial cancer survivors (ECS) in particular have favorable outcomes following primary treatment (usually surgery), with >95% of women diagnosed with localized disease surviving at 5 years post-treatment [17].
However, ECS are typically overweight or obese, eat poor diets, are inactive and report significant morbidity and reductions in QOL [4, 6, 19, 25]. Therefore, while the benefits of lifestyle changes for these women are clear, attempts to address them remain a challenge. For example, pain has been found to impact the odds of ECS completing a home-based exercise intervention [21]. A significant concern given that evidence from a recent systematic review indicates that inactivity is highly correlated with worse QOL, and even more so for obese ECS [2]. Not surprisingly, gynecologic cancer survivors most frequently report learning needs for coping with pain, daily living activities and psychosocial support [1]. Unfortunately, ECS generally do not engage in lifestyle change of their own accord [20, 25]. Compounding this problem, primary health care providers have been shown to experience their own barriers when attempting to communicate the importance of weight-loss, healthy diets and physical activity during survivorship [12].
To date only a small number of studies have examined the feasibility and preliminary efficacy of comprehensive lifestyle interventions in this population [26, 3, 16, 23]. These studies recruited between 10-19% of eligible women. The two larger trials with significantly more resources achieved rates towards to top of that range, indicating that recruiting this population of survivors is a challenge. For the potential of lifestyle interventions to be more fully realized the recruitment of representative samples of women into these trials is essential.
In 2013, the NCI and American Society of Clinical Oncology (ASCO) hosted a symposium to assess the current state of accrual science and identify new interventions to improve clinical trial enrollment [7]. Conclusions were that: 1) no rigorously tested interventions have yet addressed enrollment challenges, and 2) that enrollment needs to account for the multifactorial characteristics of the target population. The report further assessed recruiting challenges at 3 levels: the patient/community level, the clinician/provider level and the site/organizational level. A separate systematic review of 58 studies aimed at identifying barriers to completion of trials also identified “themes” related to the patient's and the health professional's perspectives[9]. From the patient perspective, treatment preference, uncertainty about benefits, timing of invitation to participate in the trial, and sociodemographic and practical barriers were listed. From the health professional's perspective, a range of systems-related and organizational barriers were identified. Engaging, recruiting and retaining representative samples is time-consuming, expensive and requires adequate planning.
Studies aiming to test novel theories, models or techniques as the foundation of behavior change must necessarily be small in scale initially. Therefore, it is vital that such studies are able to leverage their strengths by reaching as many eligible participants in as short a time as possible. These studies are also often the foundation on which young and early career scientists develop and refine their skills. Overly ambitious projects may be more effective and lead to more substantial gains if broken down into more manageable pieces, relevant to their goals. Given the time available for many small scale projects (dissertations, theses or pilot studies), trying to accomplish too much can lead to unnecessary stress on those involved and ultimately impact the scientific rigor of the studies that are done despite sound theoretical hypotheses.
In the current report we will describe the challenges faced and the lessons learned in the process of conducting a two-phase approach to studying lifestyle behaviors in a population of ECS. The purpose of this examination is to provide insight to early career scientists with regards to conducting similar studies as well as for clinicians who may also benefit from having a better understanding of the vital role they play in facilitating the conduct of research and interventions in general. Ultimately this may help to improve the quality of training and work of scientists and potentially lead to better outcomes for cancer survivors.
Methods
There were two phases to this research project to be completed in a 2 year period. The first phase; a cross-sectional survey of lifestyle behaviors was to inform the second phase—a feasibility randomized intervention study. A Pelotonia Fellowship and a Food Innovation Center (FIC) award of The Ohio State University supported this research. The cross-sectional survey study was designed to examine lifestyle behaviors in the context of psychosocial theory and mindfulness, which would then guide the design and delivery of an intervention by targeting specific correlates of physical activity and diet. We also examined aspects of. To evaluate the recruitment process we compared clinic records and enrollment data over time and location. Chi-squared tests were also used to compare the effectiveness of various recruitment strategies over time. Our protocol was approved by the Institutional Review Board and all participants provided informed consent.
Participants
Target accrual for the cross-sectional survey was 150-200 postmenopausal women who were diagnosed with type I endometrial cancer (EC). Target accrual for the intervention study was 60 (20 per group). ECS who were to be randomized into: 1) an aerobic walking intervention coupled with dietary counseling (Walk+Diet); 2) a mindfulness-based intervention coupled with dietary counseling (MIM+Diet); or 3) a usual care (UC) control group. Our target accrual was based on the ∼500 EC patients treated through our University's OBGYN clinics over 24 months identified through clinic records. We estimated that a minimum recruitment rate of 40% for the survey would provide 200 participants, which was appropriate for a structural equation modeling analysis, and a recruitment rate of 15% of the same population for the intervention would allow us to estimate effect sizes for the design of a larger trial, while also allowing us to reach our recruitment targets within the available time-frame (2 years for both studies). The detailed inclusion and exclusion criteria for both studies are reported in Table I.
Table 1. Inclusion and Exclusion Criteria.
Inclusion Criteria
|
Exclusion Criteria
|
Due to recruiting challenges for both studies and time constraints, we were unable to enroll the number of participants needed to conduct adequately powered planned analyses for the survey and decided to deliver only a single arm of the intervention—the MIM+Diet approach. This decision was based on the time left to complete the intervention study and because previous studies have examined exercise but not mindfulness-based interventions in this population. This allowed us to investigate the novel mindfulness and dietary counseling intervention, while still offering some benefit to those interested in the study.
Initial Recruitment Strategy
Eligible patients were identified from medical records prior to routine clinic visits. Primary clinicians recommended participation to eligible patients. Clinic nurses asked these patients at check-out if they were willing to speak with a researcher about aspects of their lifestyle and current quality of life. Interested patients received further details of the study and were then asked to choose a method of survey completion (a paper version with a prepaid return envelope or an online version, with the link sent to them at an email address they provided).
We did not meet our recruitment goals using this approach, which led us to also mail invitations to patients identified in clinic records between October 2011 and September 2013. Three additional iterations were made to the recruitment strategy, which are described below.
Iteration 1
The research study was introduced to patients at check-in rather than at check-out. The rationale for this change was that we could eliminate potential barriers to accrual that may have been introduced during a patient's visit (including fatigue, discussion of recurrent disease, or other distracting factors). After no noticeable improvement in recruitment we implemented iteration 2.
Iteration 2
To expand the pool of eligible patients, we contacted clinicians at two other local medical centers. We met with nursing staff and clinicians at these external sites to explain the goals of the study, the benefits and possible outcomes for their patients. After a few months with no further progress iteration 3 was implemented. Due to the limited timeframe that funding was available we simultaneously began recruitment for the lifestyle intervention at this point.
Iteration 3
We identified eligible patients previously treated through the OBGYN clinic and mailed them an invitation to participate in the survey and the intervention concurrently. We targeted all patients with an ICD 9 diagnosis of 182.0 (malignant neoplasm of corpus uteri, except isthmus). We then focused on patients with a local telephone area code, to exclude those likely too far away to travel to the study site each week.
Analyses
To determine significant differences in the response rates for both the primary sites and the method of recruitment (In-clinic vs. Mailout; Paper vs. Online), we conducted ongoing assessments of accrual. A chi-squared analysis utilizing Fisher's exact test for small samples where the expected value per approach may be <5 to compare the approach methods was used. All analyses were conducted with SPSS version 21.
Results
Initial recruitment strategy
At the patient level we did not generate sufficient interest in the survey study. Over the first 4 months we recruited 12/51 (23%) of eligible patients. Identified barriers were related to patients' understanding of the importance of lifestyle behaviors and competing concerns associated with treatment. At the clinic/provider level we identified that patients who were being approached following their clinic visit seemed to be tired, uninterested or eager to leave the clinic.
Iteration 1
Changing the offer of the study from check-out to check-in did not noticeably improve the recruitment rate over the next 2 months. In addition to the remaining time frame available, we also identified the size of the patient pool as a limitation.
Iteration 2
Approaching external clinics was well received, however at the clinic/provider level we did not have dedicated study nurses to check on this protocol at the external sites and only one patient was recruited in this manner. At the site/organizational level the time required for protocol changes to be implemented through the IRB was a barrier.
Iteration 3
We identified 438 potentially eligible patients from the local area code through clinic records from a 2-year period. At the site/organizational level only selecting patients from the local area code may have limited potential survey responses. In terms of the intervention, the type of study being offered and the change in format to a single arm study were potential barriers to participation.
Survey
For the cross-sectional survey, 511 potential participants were offered the opportunity to participate resulting in a final response rate of 13.9% (Table II) over approximately 14 months. For the entire survey, 58 (81.6%) had complete data. There was a significant association between the type of approach used (Mailout vs. In-clinic) and whether patients responded “yes” to the offer of the survey (χ2 (1) = 41.556). Based on the calculated odds ratio, individuals were 81% more likely to refuse the survey when it was offered via mailout compared with when it was offered in-clinic. Standardized residuals and z-tests showed that significantly fewer people (-2.8) or a lower proportion (49.3%) than expected said “yes” to the mailout.
Table II. Recruitment Characteristics.
| Study | Total Possible | Actual Responses | Response Rate |
|---|---|---|---|
| Survey Study | |||
| In clinic | 110 | 36* | 32.7% |
| Mailout | 401 | 35 | 8.7% |
| Total | 511 | 71 | 13.9% |
|
| |||
| Intervention Study | |||
| In clinic | 37 | 3 | 11.0% |
| Mailout | 401 | 40 | 10.7% |
| Total | 438 | 43# | 9.8% |
Note
52% online, 48% paper,
17 eligible (39.5%)
Standardized residuals showed that significantly more people said “yes” (5.3) to the in-clinic survey and significantly fewer said “no” than expected (-2.1) while z-tests showed that a significantly greater proportion of people responded “yes” (50.7%) than responded “no” (16.8%) to in-clinic offers. There were no other differences between these groups. Finally, for women approached in-clinic, 52% responded online, while 48% responded via paper. Women could only respond via an online link when we mailed out invitations to the survey (9%).
Intervention
For the MIM+Diet intervention, 43 (9.8%) of 438 patients approached showed an interest in the intervention. Of these, 26 were excluded (see Figure I). A further 9 patients withdrew, either because the times offered for the intervention were inconvenient or because of the chance of being randomized into a group they did not want. This resulted in 17 of 43 (40%) participants completing baseline assessments. Over the 14-week intervention, 4 were lost to follow-up. Those who participated attended 86 out of a possible 104 sessions (83%).
Figure 1. CONSORT Diagram for Participant Flow.
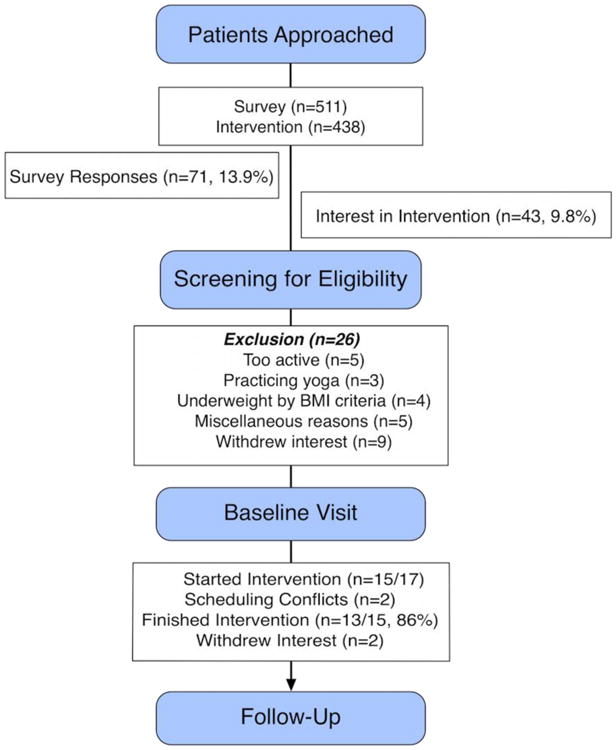
When examining the two approaches to recruiting patients to the intervention (Mailout vs. In-Clinic), one expected frequency was lower than 5 (3.6), and thus we used Fisher's exact test for analysis. We found no significant associations between the type of approach used and whether patients showed an interest in the intervention (p=0.496).
The in-clinic approaches were made during a single day of the week over 14 months (∼56 weeks), whereas the mailouts required at most a few weeks to complete. The in-clinic approach was time-consuming and labor-intensive. Despite a better response rate to the survey study when offered to patients in-clinic, there was no difference when the intervention study was offered in-clinic compared to mail.
Discussion
In the process of recruiting ECS to two research studies focused on lifestyle behaviors we encountered significant challenges. We recruited 13.9% of eligible patients to the survey study and 4% of patients who were contacted about the intervention study. The slower than expected accrual meant that we were unable to conduct robust analyses of the hypothesized theoretical correlates of lifestyle behaviors in ECS. Further, we were unable to examine the feasibility and preliminary efficacy of the traditional walking and dietary counseling (Walk+Diet) as compared to a mindfulness and dietary counseling (MIM+Diet) and usual care (UC) intervention arms. The challenges faced and lessoned learned in this process may prove as a useful guide for early career scientists and clinical staff involved in the conduct of behavioral research. We focus part of our discussion on the recruiting challenges of these studies within the framework and recommendations made in the NCI/ASCO report [7], published following completion of our studies. The report focused on the patient/community level, the clinician/provider level and the site/organizational level. We also discuss the needs of ECS and the unique challenges they face.
Previous evidence has pointed to the difficulty of recruitment to well-funded large scale clinical trials [10]. Here we are focused on breaking down the challenges faced during the initial stages of concept development and feasibility testing in two smaller research projects. Given the number of patients seen during a calendar year in our large university clinic (∼250-300) it was expected that the relatively small target sample would be easily met. For comparison, the Alberta Cancer Registry documented 529 new cases of uterine cancer in 2012. Using the Alberta Cancer Registry, Courneya and colleagues [6] mailed out surveys to endometrial cancer patients in order to examine the relationships between exercise, body weight and quality of life. A total of 248 oncologists were contacted for permission to approach their 1549 ECS, and 198 responses from oncologists (79.8%) resulted in mailouts to 879 patients, or just over 55%. A final response was received from 386 patients out of 769 (50%). That study used a slightly modified version of the total design method [8], which consists of the first mailing followed by a postcard reminder 2 weeks later, and a final mailing 4 weeks after that. Other techniques that were employed included a personalized letter, color printed questionnaires, original signatures, assurances of confidentiality and institution sponsorship. The benefits of using a cancer registry are that an established system of identifying and approaching patients can lead to increased acceptance of the research process by patients and a reduction in the burden on the researchers to identify and contact eligible individuals. A prior feasibility intervention study designed to investigate dietary and exercise-related lifestyle behaviors in ECS achieved a 29% recruitment rate, with 84% of patients completing follow-up [24]. In that study, investigators initially mailed a letter to cancer patients, also identified through a registry over a 3-year period, inviting them to an information session. The greater success rate in these previous studies highlights the benefits of using a registry and the potential of the mailing approach, however given the scale of our studies we were unable to conduct as complete and rigorous a strategy over as long a period of time.
Specific challenges faced, lessons learned and recommendations
We did our best to plan our studies by consulting previous literature, speaking with clinicians and taking into account the time required to progress from phase 1(survey) to phase 2 (intervention). However, the limited experience of recruiting this particular population to research focused on lifestyle behaviors and limited resources in terms of both funding and personnel meant we were not able to adequately respond to the recruiting challenges that we faced.
Patient/community level
ECS are particularly difficult to recruit suggesting that as a population, they do not see their condition as especially threatening and are therefore not motivated to change their behaviors. Many ECS are given a favorable prognosis and they may therefore be less motivated to participate in clinical research studies than those with a poor prognosis. A poor understanding of the risks of disease recurrence and for developing comorbidities associated with poor lifestyle behaviors may contribute to ECS's apathy towards change. In contrast, breast cancer survivors as a group are seen as being highly motivated and may therefore be more commonly recruited onto trials. Furthermore, while breast cancer patients are often overweight, ECS are typically obese. Obesity may be a specific barrier to recruiting ECS onto any trial but may make it particularly difficult to engage in lifestyle interventions; many overweight and obese patients are limited in their capacity to exercise [25]. Other potential issues affecting recruitment may include the stigma associated with EC diagnosis, the experience of treatment-related side effects and embarrassment regarding their functional capacity. Importantly, ECS also frequently report the need for resources to help them deal with pain [1] and that pain can impact their ability to remain in lifestyle studies [21]. Interestingly, a recent study found ECS who were exercisers had lower levels of co-morbidity and were more likely to report that feeling better physically and emotionally rather than reducing the risk of disease was the important outcome of exercise[13], which may be useful for care providers trying to educate these women in future approaches.
We offered women a healthy lifestyle behavior program that included physical activity, diet and stress management. This may have made our study less attractive to potential participants for all the reasons mentioned above, making recruitment even more difficult. Further, we ultimately chose to focus on a single arm of the originally proposed intervention— the MIM+Diet arm. While we did not specifically change recruitment materials to specify this change, patients could have been even less interested in this option. In future it may be important to step back and understand the patients perspectives and their unique needs and concerns, which may have helped to inform the design of the intervention and improved recruitment.
The guidelines recommended in the NCI/ASCO report include consideration of the patient point of view, addressing reasons why patients do not participate, simplification of informed consent, education of patients and community about clinical trials, involvement of advocates, engaging racial and ethnic minorities, involving community leaders in the design of trials, use of social media, registries and databases especially for quality of life and survivorship studies. While the scale of our studies precluded us from addressing all of these recommendations we did use a local patient database, and we considered many of the unique characteristics of our population, such as age, technological experience and geographic location. A primary limitation of our approach was the lack of an assessment of why patients did not want to participate in our studies. Unfortunately we did not expect to face recruiting challenges and hence did not survey patients about why they were not interested in participating. By the time we identified this as an option we were concerned the delays through IRB would further limit recruitment. For future studies we also recommend the use of a community advocate or using a community-based approach, which may help with recruiting minorities. Social media may also be an effective way to advertise a study, however older patients may not be well targeted in this manner and recent research suggests the effectiveness of this approach remains undetermined [22]. Finally, in future, it may be appropriate to offer a small incentive to improve accrual rates. For these studies, we were partly limited by budget and partly based on the fact that we were measuring motivation as a construct, which we felt could have been influenced by the promise of remuneration.
Clinician/provider level
NCI-ASCO recommendations at this level include developing evidence based training for provide-patient communication, providing incentives for clinicians to participate in research, disseminating information to local providers about trial availability, recruiting investigators from underserved communities, speaking with referring clinicians, recruitment planning with research teams, use of registries and publishing on successful/unsuccessful strategies. Research indicates that clinicians are one of the most important factors influencing accrual to trials [11, 18]. We did consult with both physicians and nurses treating EC patients. While we had strong engagement by these clinicians, there was no formal system or training to educate and involve nurses and clinic staff about the goals of our study and how to communicate this to patients. Providing nurses and clinical staff with the opportunity to be directly involved in the planning of the research process may have provided them with a sense of control over their workload and further engaged their interest. Identifying the “gatekeepers” of access to patients early—often research nurses —is critical to the success of any research study. Implementing regular planning meetings to assess progress and providing feedback to these staff could also have helped to foster involvement in our studies.
Site/organizational level
These smaller scale research studies did not directly engage at the research site or organizational level initially. Recommendations at this level include promoting accrual through leadership best practices, implementing site and trialist performance standards, using screening logs to verify patient availability, promoting ownership of investigator initiated trials, use of formal quality improvement techniques, use of clinical trials management system, closing of trials with slow accrual and using IRB shortening practices. In the later stages of the project the clinical trials office was consulted to try and increase awareness of the research and to request that research nurses recommend the study to patients. A lack of institutional support including insufficient staffing of already busy research nurses and support staff is critical. The small scale of our studies meant it was likely low priority when compared with the large scale well-funded trials being conducted at the institution. However, earlier engagement within this organizational level during the planning phase would have likely resulted in better support. IRB shortening practices may have also allowed more flexibility in our responses to recruitment providing us with more time, this is rarely easy to navigate and again adequate planning is likely more feasible.
Other recommendations
In addition to the literature, researchers should directly consult with investigators outside their institution who have experience specific to the population and even the behaviors/outcomes in question if relevant. Many research papers do not report on specific challenges and their potential solutions; therefore in many cases researchers are reinventing the wheel, especially young investigators. However, it should also be recognized that each research environment while having similarities to others may also be unique.
In-Clinic vs. Mailout
We found a greater than expected rate of response to our survey for in-clinic approaches when compared with mail-out. One potential explanation for this finding lies in the value of face-to-face contact among nursing staff, researchers, and potential participants. For example, while more patients can be contacted via mail, they cannot be asked follow-up questions or clarify study goals, risks and expected outcomes. Ideally, multiple approaches should be used; e.g., inviting potential participants to attend information sessions or to speak with investigators has been shown to be effective[26].
Online vs. Paper
An initial goal of the survey study was to evaluate the efficacy of a novel smart device/tablet-based survey approach. The survey was to be delivered during the time patients typically wait for their clinic visit. However, most of the older female patients were not comfortable completing the survey using this technology. We therefore provided a paper version that could be mailed back, or asked if interested patients could provide an email to which a link to the survey could be emailed. The email option allowed us to track responses and send follow-up prompts. As technology continues to become integrated into patient care approaches, it is likely that a larger proportion of patients will opt for this method of contact in future studies.
Significant time requirements and the expense and difficulty of travel have prompted some investigators to evaluate the feasibility of home-based lifestyle interventions. Basen-Enquist and colleagues [3] used a social cognitive theory-based approach to improve exercise levels in ECS. While this larger well-funded study also reported recruitment challenges [15], this option is an important consideration, especially if the target population is spread out geographically. In our own study, we screened patients based on their area code to eliminate this problem as much as possible.
Conclusions
It is essential to educate researchers, clinicians and their staff about the importance and specific challenges related to recruitment to research studies. Similarly, it is essential for clinicians to educate their patients about the importance of participating in research regarding behavioral approaches. Without their clinician's support, patients will not appreciate the benefits of lifestyle changes. Likewise, without patients' support, clinicians may not be convinced of the interest or likelihood of patients to benefit from such research. People involved in all levels of the research process from the research lab to the bedside should be offered the opportunity to contribute to the planning and development of research studies in order to improve their conduct and ultimately the quality and validity of their results. At the early career-stage careful planning is required to make the best use of available time and resources, especially in weighing up the benefits vs. challenges likely to be encountered while conducting preliminary work.
Acknowledgments
This work was supported by NIH/NCI 5R25 CA122061 a Food Innovation Center (FIC) award of The Ohio State University and the Pelotonia Fellowship Program. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the Pelotonia Fellowship Program.
References
- 1.Akkuzu G, Kurt G, Guvenc G, Kok G, Simsek S, Dogrusoy S, Ayhan A. Learning Needs of Gynecologic Cancer Survivors. J Cancer Educ. 2016:1–7. doi: 10.1007/s13187-016-1118-y. [DOI] [PubMed] [Google Scholar]
- 2.Babatunde OA, Adams SA, Orekoya O, Basen-Engquist K, Steck SE. Effect of Physical Activity on Quality of Life as Perceived by Endometrial Cancer Survivors: A Systematic Review. Int J Gynecol Cancer. 2016;26(9):1727–1740. doi: 10.1097/IGC.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 3.Basen-Engquist K, Carmack CL, Li YS, Brown J, Jhingran A, Hughes DC, Perkins HY, et al. Social-Cognitive Theory Predictors of Exercise Behavior in Endometrial Cancer Survivors. Health Psychol. 2013;32(11):1137–1148. doi: 10.1037/a0031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, Hughes D, Taylor CC. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200(3):288 e281–288. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer. 2007;17(2):441–446. doi: 10.1111/j.1525-1438.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 6.Courneya KS, Karvinen KH, Campbell KL, Pearcey RG, Dundas G, Capstick V, Tonkin KS. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, Dilts DM, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: Summary and Recommendations. J Oncol Pract. 2013;9(6):267–276. doi: 10.1200/jop.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillman Don A. Mail and telephone surveys. Wiley Interscience; 1978. [Google Scholar]
- 9.Fayter Debra, McDaid Catriona, Eastwood Alison. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol. 2007;60013(10):990.e991–990.e933. doi: 10.1016/j.jclinepi.2006.12. [DOI] [PubMed] [Google Scholar]
- 10.Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs. 2010;19(1-2):227–233. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Keating NL, Potosky AL, Ambs A, He YL, Hornbrook MC, Ganz PA. A Population-Based Assessment of Specialty Physician Involvement in Cancer Clinical Trials. J Natl Cancer Inst. 2011;103(5):384–397. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laskey RA, McCarroll ML, von Gruenigen VE. Obesity-related endometrial cancer: an update on survivorship approaches to reducing cardiovascular death. Br J Obstet Gynaecol. 2016;123(2):293–298. doi: 10.1111/1471-0528.13684. [DOI] [PubMed] [Google Scholar]
- 13.Lukowski J, Gil KM, Jenison E, Hopkins M, Basen-Engquist K. Endometrial cancer survivors' assessment of the benefits of exercise. Gynecol Oncol. 2012;124(3):426–430. doi: 10.1016/j.ygyno.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Maddams J, Brewster D, Gavin A, Steward J, Elliott J, Utley M, Moller H. Cancer prevalence in the United Kingdom: estimates for 2008. Br J Cancer. 2009;101(3):541–547. doi: 10.1038/sj.bjc.6605148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin Michelle Y, Pisu Maria, Manne Sharon, Basen-Engquist Karen, Alfan Catherine. Ann Behav Med. Springer; 233 Spring St, New York, NY 10013 USA: 2014. To Evaluate Efficacy, We Need to Reach: How do We Recruit Cancer Survivors and Family Members to Psychosocial Research Studies? [Google Scholar]
- 16.McCarroll ML, Armbruster S, Pohle-Krauza RJ, Lyzen AM, Min S, Nash DW, Roulette GD, Andrews SJ, von Gruenigen VE. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. 2015;137(3):508–515. doi: 10.1016/j.ygyno.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 18.Minasian LM, O'mara AM. Accrual to Clinical Trials: Let's Look at thePhysicians. J Natl Cancer Inst. 2011;103(5):357–U1500. doi: 10.1093/jnci/djr018. [DOI] [PubMed] [Google Scholar]
- 19.Modesitt SC, Geffel DL, Via J, Weltman AL. Morbidly obese women with and without endometrial cancer: Are there differences in measured physical fitness, body composition, or hormones? Gynecol Oncol. 2012;124(3):431–436. doi: 10.1016/j.ygyno.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Soliman PT, Bassett RL, Wilson EB, Boyd-Rogers S, Schmeler KM, Milam MR, Gershenson DM, Lu KH. Limited public knowledge of obesity and endometrial cancer risk - What women know. Obstet Gynecol. 2008;112(4):835–842. doi: 10.1097/Aog.0b013e318187d022. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Karlsten M, Yamal JM, Basen-Engquist K. Health-related quality of life factors associated with completion of a study delivering lifestyle exercise intervention for endometrial cancer survivors. Qual Life Res. 2016:1–9. doi: 10.1007/s11136-016-1441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topolovec-Vranic Jane, Natarajan Karthik. The Use of Social Media in Recruitment for Medical Research Studies: A Scoping Review. J Med Internet Res. 2016;18(11):e286. doi: 10.2196/jmir.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109(1) doi: 10.1016/j.ygyno.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 24.von Gruenigen Vivian E, Courneya Kerry S, Gibbons Heidi E, Kavanagh Mary Beth, Waggoner Steven E, Lerner Edith. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: A randomized trial. Gynecol Oncol. 2008;109(1):19–26. doi: 10.1016/j.ygyno.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 25.von Gruenigen Vivian E, Waggoner Steven E, Frasure Heidi E, Kavanagh Mary Beth, Janata Jeffrey W, Rose Peter G, Courneya Kerry S, Lerner Edith. Lifestyle Challenges in Endometrial Cancer Survivorship. Obstet Gynecol. 2011;117(1):93–100. doi: 10.1097/AOG.0b013e31820205b3. [DOI] [PubMed] [Google Scholar]
- 26.von Gruenigen Vivian, Frasure Heidi, Kavanagh Mary Beth, Janata Jeffrey, Waggoner Steven, Rose Peter, Lerner Edith, Courneya Kerry S. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): A randomized controlled trial. Gynecol Oncol. 2012;125(3):699–704. doi: 10.1016/j.ygyno.2012.03.042. [DOI] [PubMed] [Google Scholar]


