Abstract
Background
Trends in the prevalence of chronic kidney disease (CKD) are important for health-care policy and planning.
Objective
To update trends in CKD prevalence.
Design
Repeated cross-sectional study.
Setting
National Health and Nutrition Examination Survey (NHANES) in 1988–94 and every two years from 1999 to 2012.
Participants
Adults 20 years or older.
Measurements
CKD (stages 3–4) was defined using glomerular filtration rate (GFR) estimated with the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation from calibrated serum creatinine measurements (eGFR 15–59 ml/min/1.73m2). An expanded definition of CKD also included persons with an eGFR ≥60 ml/min/1.73m2 and a one-time urine albumin-to-creatinine ratio ≥30 mg/g.
Results
An increase in the unadjusted prevalence of stages 3–4 CKD occurred from the late 1990s to the early 2000’s. Since 2003–04, however, the prevalence of stages 3–4 CKD overall has largely stabilized (e.g. 6.9% prevalence of stage 3–4 CKD in 2003–04 and 6.9% prevalence in 2011–12). There was little difference in adjusted prevalence of stage 3–4 CKD overall comparing 2003–04 and 2011–12 after controlling for age, sex, race/ethnicity, and diabetes mellitus status (p=0.26). Lack of increase in CKD prevalence since the early 2000’s was observed in most subgroups and with an expanded definition of CKD which included persons with higher eGFRs but with albuminuria.
Limitations
Serum creatinine and albuminuria were measured only once in each subject.
Conclusions
In a reversal of prior trends, there has been no appreciable increase in the prevalence of stages 3–4 CKD in the U.S. population overall during the most recent decade.
INTRODUCTION
Much attention has been paid over the last four decades to the enlarging size of the end-stage renal disease (ESRD) population. This is well-documented by comprehensive national registries such as the United States Renal Data System (USRDS)(1). Recently there has been a notable change in the epidemiology of ESRD, with decreasing adjusted incident rates of ESRD. Specifically, the age-sex-race/ethnicity-adjusted ESRD incidence rate in the United States was 386 cases per million/year in 2003 but 356, 352 and 351 cases per million/year in 2011, 2012 and 2013 respectively (1).
Almost all cases of ESRD are preceded by a period of chronic kidney disease (CKD)(2, 3). Given this natural history, an antecedent change in CKD epidemiology in the United States prior to substantial change in ESRD epidemiology would be expected. Yet, the existing peer-reviewed publications have mostly reported that the overall prevalence of CKD in the U.S. population had been increasing (4), by as much as up to 5% a year (5, 6). An important limitation in the peer-reviewed literature is that CKD prevalence in more recent years has not been analyzed (4, 5, 7–9).
Separate from it being a precursor to ESRD, CKD is now recognized as an important risk factor for other adverse outcomes such as acute kidney injury, cardiovascular disease, and premature death (10–12). The prevalence of CKD is one to two orders of magnitude higher than the prevalence of ESRD (13). CKD epidemiology in its own right has received much attention over the last dozen years and has been incorporated into programs of nationwide health-promotion and disease-prevention goals (2, 3, 14). For example, the U.S. Department of Health and Human Services Healthy People 2020 has set a target of 10 percent proportional reduction in CKD prevalence in the U.S. population (14).
The goal of the present study is to provide updated estimates of CKD prevalence in the United States. We analyzed National Health and Nutritional Examination Survey (NHANES) from 1988–1994 through 2011–2012 to estimate the temporal trend of CKD prevalence overall and in subgroups of the population, with particular attention to more recent years.
METHODS
Study Population
The NHANES is a nationally-representative survey of non-institutionalized, US civilian residents that is conducted by the National Center for Health Statistics and involves a combination of in person interviews, physical examination, and laboratory data (15). NHANES III was conducted 1988–1994 and beginning in 1999 NHANES collected data continuously and released datasets in 2 year data cycles. We examined data from NHANES III (1988–94) through NHANES 2011–12. We limited our study to participants aged 20 years or older with available serum creatinine measurements. For the current analysis, we included only participants seen in the mobile examination centers where comparable laboratory measurements were taken. Patients with estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2, corresponding to stage 5 CKD, were excluded due to small sample size and inability to determine if these persons were on maintenance dialysis or not.
Measures of Kidney Function
Our main outcome of interest was the prevalence of stages 3–4 CKD (as defined by eGFR of 15–59 mL/min/1.73m2 (16, 17)) in each cycle of NHANES. In the primary analysis, eGFR was determined by the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation (18). We used the NHANES recommended calibrations for serum creatinine measurements across time periods (15)(Supplemental Table 1). In sensitivity analysis, calculations were repeated with the Modification of Diet in Renal Disease study (MDRD) eGFR equation (19).
In secondary analysis, we used an expanded definition of CKD to include persons with an eGFR ≥60 ml/min/1.73m2 and a one-time urine albumin-to-creatinine ratio (ACR) ≥30 mg/g. We did not attempt to define persistent proteinuria, given that most persons only had one measurement in the survey, and given that timing of second urine collection varied (e.g. first morning void vs. random) when albuminuria was repeated during selected cycles of NHANES or in selected subsamples (4, 20).
Demographics and Comorbidities
NHANES collected data on participant demographics, including age, sex, and race/ethnicity. To ensure consistency across all NHANES cycles, we classified individuals as being non-Hispanic white, non-Hispanic black, and other race/ethnicity. During the physical exam portion of the survey, measurements including height, weight, blood pressure, and other parameters were assessed. Blood and urine samples are taken, which allowed measurement of hemoglobin A1c (A1c) in addition to serum creatinine and urine ACR. Diabetes mellitus (hereafter called diabetes) was defined in this study as a self-reported physician diagnosis, use of diabetes medication (i.e., oral hypoglycemic medications or insulin), or a laboratory measured A1c ≥6.5% (21).
Statistical Analysis
This analysis used the recommended NHANES examinations sample weights (22). Data were analyzed using the SURVEYLOGISTIC procedure in SAS to accommodate the complex sample survey design (23). 95% confidence intervals were generated for crude prevalences.
In adjusted analyses, restricted cubic splines were used to flexibly model the trends over time. Three knots were used at pre-determined locations of years 2000, 2004 and 2007 to both give the needed flexibility in shape and to allow changes over the time periods of interest. Since we were particularly interested in updating trends in CKD prevalence, we a priori chose to compare prevalence from NHANES 2003–04, which was the data cycle used for the most recent prior analyses, (4, 5, 7–9) to the latest NHANES 2011–12. To display temporal trends or temporal trends stratified by a factor (e.g., age), we used marginal effect estimation (24). For example, to display the effect of age over time, marginal effect estimation holds the other covariates (sex, race/ethnicity, and diabetes status) fixed at their observed values while varying age and year. It does this by using the fitted model to calculate the predicted risk for each year and each person as if the person were in each of the different age categories; those are then averaged to calculate the average CKD risk for a given age and year. We used the standard Wald tests from Stata’s sample survey logistic regression analysis (which derive standard errors using Taylor linearization) to derive p-values. To compare the prevalence in 2003–4 with prevalence in 2011–12 we used the “lincom” command in Stata to compare the heights of the curve at the two years. To test for interactions of the temporal trends across categories (e.g., age) we tested the combined interaction of the spline terms with the category of interest. Values for graphing the spline fits and their standard errors were derived using the “margins” command in Stata. Analyses were performed using SAS Ver 9.2 (SAS Institute, Cary, NC) and Stata 13.1 (StataCorp, College Station, TX).
Role of the Funding Source
The ASN and the NIH had no role in the design, conduct, and analysis of the study or in the decision to submit the manuscript for publication. National Center for Health Statistics/CDC authors provided technical advice and manuscript review. The CDC performed a final review of the manuscript to ensure that the analysis met methodological standards. The investigators are solely responsible for the content and the decision to submit the manuscript for publication.
IRB Approval
This study is approved by the University of California, San Francisco Institutional Review Board (Committee on Human Research application # 10-04162).
RESULTS
Characteristics of adult U.S. population we studied by survey periods are shown in Table 1. In later calendar years, mean age was higher, the percentage of non-Hispanic whites was lower and a higher fraction of the population was classified as having diabetes mellitus.
Table 1.
Characteristics of U.S. adults included in study by National Health and Nutritional Examination Survey (NHANES) survey period (1988–2012)
| 1988–1994 (n=15484) | 1999–2000 (n=4099) | 2001–2002 (n=4684) | 2003–2004 (n=4448) | 2005–2006 (n=4451) | 2007–2008 (n=5303) | 2009–2010 (n=5662) | 2011–2012 (n=4869) | |
|---|---|---|---|---|---|---|---|---|
| Mean age (±SE), years | 44.8 ± 0.46 | 44.9 ± 0.38 | 45.4 ± 0.53 | 46.4 ± 0.50 | 46.7 ± 0.73 | 47.0 ± 0.42 | 47.0 ± 0.50 | 47.3 ± 0.81 |
|
|
||||||||
| Female sex (±SE), % | 52.2 ± 0.37 | 52.0 ± 0.48 | 51.9 ± 0.45 | 51.5 ± 0.73 | 51.9 ± 0.64 | 51.7 ± 0.67 | 51.6 ± 0.66 | 51.7 ± 0.76 |
|
|
||||||||
| Race/ethnicity (±SE), % | ||||||||
|
|
||||||||
| Non-Hispanic white | 76.9 ± 0.54 | 70.8 ± 0.69 | 72.3 ± 0.70 | 72.5 ± 1.17 | 72.5 ± 1.03 | 70.1 ± 1.10 | 68.9 ± 1.07 | 67.4 ± 1.25 |
|
|
||||||||
| Non-Hispanic black | 10.3 ± 0.56 | 10.3 ± 1.47 | 10.1 ± 1.39 | 10.8 ± 1.38 | 11.0 ± 1.44 | 10.2 ± 1.51 | 10.6 ± 1.03 | 10.8 ± 1.67 |
|
|
||||||||
| Other race/ethnicity | 12.8 ± 0.47 | 18.8 ± 1.57 | 17.6 ± 1.58 | 16.7 ± 0.92 | 16.6 ± 0.88 | 19.6 ± 1.11 | 20.5 ± 1.35 | 21.9 ± 1.20 |
|
|
||||||||
| With diabetes mellitus (±SE), % | 7.4 ± 0.49 | 7.6 ± 0.92 | 8.3 ± 0.79 | 9.8 ± 1.38 | 9.6 ± 0.92 | 11.3 ± 1.27 | 10.9 ± 0.90 | 11.5 ± 1.30 |
|
|
||||||||
| Mean serum creatinine concentration (±SE), mg/dl | 0.84 ± 0.00 | 0.88 ± 0.00 | 0.89 ± 0.01 | 0.89 ± 0.00 | 0.89 ± 0.01 | 0.87 ± 0.01 | 0.88 ± 0.00 | 0.87 ± 0.01 |
|
|
||||||||
| Mean urine albumin-to-creatinine ratio (ACR) (±SE), mg/g | 25.3 ± 1.57 | 29.8 ± 5.48 | 29.5 ± 2.58 | 23.2 ± 2.80 | 31.2 ± 3.20 | 37.1 ± 4.17 | 23.1 ± 2.60 | 25.0 ± 2.08 |
Temporal trends in crude prevalence of CKD stages 3–4
The crude prevalence of CKD stages 3 and 4 (CKD-EPI equation estimated glomerular filtration rates [eGFR] 15–59 ml/min/1.73m2) rose from 4.8% (95% confidence interval [CI] 4.3–5.4%) in 1988–94 to 6.9% (5.9–7.9%) in 2003–04 but has largely stabilized thereafter, being 6.9% (5.5–8.3%) also in 2011–12 (Table 2)(Supplemental Table 2 shows number of NHANES participants providing data for each of the cells).
Table 2. Prevalence (percentage) of CKD stages 3–4 (eGFR 15–59 ml/min/1.73m2 by CKD-EPI equation) in U.S. adults by demographic and diabetic categories, NHANES 1988–1994 through 2011–2012;
95% confidence interval of prevalence (as percentage) are shown in parentheses; N represents the population number in 100,000’s
| Years | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1988–1994 | 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | |
|
| ||||||||
| Total Population
|
4.8% (4.3–5.4%) N = 81.24 |
5.3% (4.4–6.3%) N = 95.9 |
6.4% (5.6–7.2%) N = 120 |
6.9% (5.9–7.9%) N = 135 |
6.7% (5.2–8.3%) N = 133 |
6.5% (5.5–7.5%) N = 131 |
6.4% (5.4–7.4%) N = 132 |
6.9% (5.5–8.3%) N = 144 |
| Age | ||||||||
|
|
||||||||
| 20–39 years* | 0.1% (0.02–0.2%) | 0.4% (0.0–0.9%) | 0.2% (0.0–0.4%) | 0.2% (0.0–0.5%) | 0.1% (0.0–0.2%) | 0.3% (0.0–0.6%) | 0.1% (0.0–0.4%) | 0.2% (0.0–0.4%) |
|
|
||||||||
| 40–64 years | 2.0% (1.6–2.4%) | 2.1% (1.3–2.8%) | 3.0% (1.8–4.1%) | 3.1% (2.2–3.9%) | 3.2% (1.9–4.5%) | 3.1% (1.9–4.3%) | 2.6% (1.8–3.4%) | 3.8% (2.2–5.5%) |
|
|
||||||||
| 65–79 years | 19.4% (17.0–21.8%) | 21.8% (17.4–26.2%) | 24.6% (21.6–27.5%) | 25.1% (20.7–29.5%) | 22.4% (19.3–25.5%) | 23.3% (20.3–26.3%) | 22.3% (19.5–25.2%) | 21.7% (18.0–25.4%) |
|
|
||||||||
| ≥80 years | 43.2% (39.4–47.0%) | 53.0% (42.1–63.9%) | 59.8% (52.8–66.9%) | 56.1% (51.0–61.2%) | 55.5% (48.5–62.4%) | 47.5% (40.3–54.8%) | 50.7% (46.4–55.0%) | 51.1% (44.21–57.89%) |
|
|
||||||||
| Sex | ||||||||
|
|
||||||||
| Male | 4.1% (3.4–4.8%) | 4.3% (3.5–5.1%) | 5.4% (4.6–6.3%) | 5.7% (4.8–6.5%) | 6.0% (4.4–7.5%) | 5.0% (4.0–6.0%) | 5.3% (4.2–6.5%) | 5.9% (4.5–7.2%) |
|
|
||||||||
| Female | 5.6% (4.7–6.4%) | 6.3% (4.9–7.8) | 7.3% (6.3–8.2%) | 8.1% (6.8–9.4%) | 7.4% (5.3–9.6%) | 7.9% (6.4–9.5%) | 7.4% (6.3–8.5%) | 7.8% (6.3–9.4%) |
|
|
||||||||
| Race/ethnicity | ||||||||
|
|
||||||||
| Non-Hispanic white | 5.4% (4.7–6.2%) | 5.8% (4.5–7.1%) | 7.5% (6.7–8.4%) | 7.9% (6.7–9.0%) | 8.1% (6.3–9.9%) | 7.8% (6.5–9.0%) | 7.5% (6.3–8.7%) | 8.0% (6.1–10.0%) |
|
|
||||||||
| Non-Hispanic black | 3.7% (3.2–4.2%) | 5.3% (3.9–6.7%) | 5.2% (3.8–6.6%) | 4.9% (3.5–6.2%) | 5.3% (3.4–7.3%) | 5.5% (3.9–7.0%) | 5.9% (4.5–7.3%) | 6.2% (4.7–7.7%) |
|
|
||||||||
| Other race/ethnicity* | 2.2% (1.1–3.3%) | 3.8% (1.5–6.1%) | 2.3% (0.5–4.2%) | 4.3% (2.7–5.9%) | 1.7% (0.9–2.6%) | 2.5% (1.5–3.4%) | 3.0% (2.0–3.9%) | 3.6% (2.7–4.6%) |
|
|
||||||||
| Diabetes status | ||||||||
|
|
||||||||
| With diabetes mellitus | 14.3% (12.0–16.7%) | 14.7% (11.9–17.6%) | 16.4% (12.3–20.4%) | 19.5% (15.5–23.5%) | 19.0% (14.6–23.4%) | 16.0% (13.0–19.1%) | 19.8% (16.6–23.1%) | 19.1% (15.8–22.4%) |
|
|
||||||||
| Without diabetes mellitus | 4.1% (3.5–4.6%) | 4.6% (3.5–5.7%) | 5.5% (4.7–6.3%) | 5.6% (4.5–6.6%) | 5.4% (3.9–7.0%) | 5.3% (4.3–6.2%) | 4.7% (4.0–5.5%) | 5.3% (3.9–6.7%) |
estimates in this row have large relative standard error (RSE) and thus may be less reliable
CKD=chronic kidney disease
CKD-EPI = Chronic Kidney Disease-Epidemiology Collaboration
eGFR = estimated glomerular filtration rate
NHANES = National Health and Nutritional Examination Survey
Absolute disease prevalence was considerably higher among the older adults than the younger adults. But this lack of increase in CKD prevalence over the most recent decade was generally seen in all age strata. For example, among those age 65–79 years, the prevalence rose from 19.4% (17.0–21.8%) in 1988–94 to 25.1% (20.7–29.5%) in 2003–04 and subsequently declined to 21.7% (18.0–25.4%) in 2011–12. For both men and women, the unadjusted prevalence of CKD was higher in 2003–04 than in 1988–94 but has since largely remained unchanged: 7.8% (6.3–9.4%) in women and 5.9% (4.5–7.2%) in men in 2011–12. The crude CKD prevalence among non-Hispanic whites peaked at 8.1% (6.3–9.9%) in 2005–06 from 5.4% (4.7–6.2%) in 1988–94 and then has largely remained stable, with prevalence of 8.0% (6.1–10.0%) in 2011–12. However, the crude CKD prevalence among non-Hispanic blacks increased progressively across the study period, growing from 3.7% (3.2–4.2%) in 1988–94 to 4.9% (3.5–6.2%) in 2003–4 to 6.2% (4.7–7.7%) by 2011–12.
When the study sample was classified by diabetes status, the prevalence of CKD in the diabetes category rose from 14.3% (12.0–16.7%) in 1988–94 to 19.5% (15.5–23.5%) in 2003–04 and remained largely stable thereafter, being 19.1% (15.8%–22.4%) in 2011–12. The prevalence of CKD in the non-diabetic category rose from 4.1% (3.5–4.6%) in 1988–94 to 5.6% (4.5–6.6%) in 2003–04 and then remained largely stable, being 5.3% (3.9–6.7%) in 2011–12.
Temporal trends in adjusted prevalence of CKD stages 3–4
The adjusted trends were consistent with the crude prevalence results. There was little difference in adjusted prevalence of CKD stages 3–4 overall between 2003–04 and 2011–12 (p=0.26).
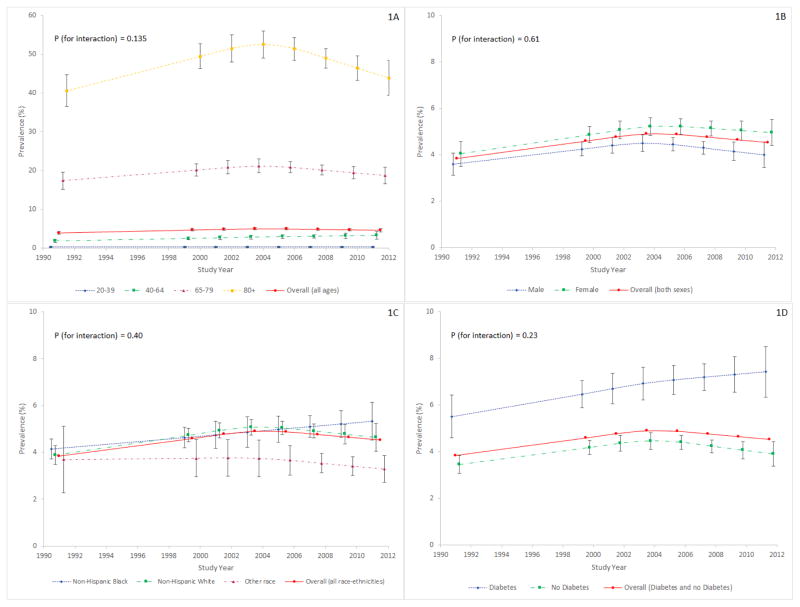
The sex, race/ethnicity and diabetes status adjusted prevalence of stage 3–4 CKD was higher among older individuals. However, in all age groups, the temporal trend was one of no further increase in adjusted prevalence after the early 2000’s (p for age-year interaction =0.135)(Figure 1A).
Figure 1. Adjusted prevalence (as percentage) of CKD stages 3–4 (eGFR 15–59 ml/min/1.73m2 by CKD-EPI equation) in U.S. adults by age (1A), sex (1B), race/ethnicity (1C) and presence or absence of diabetes mellitus (1D), NHANES 1988–1994 through 2011–2012.
Each subgroup is adjusted for the other three subgroup variables (e.g. 1A is adjusted for sex, race/ethnicity, and diabetes status).
CKD=chronic kidney disease
CKD-EPI = Chronic Kidney Disease-Epidemiology Collaboration
eGFR = estimated glomerular filtration rate
NHANES = National Health and Nutritional Examination Survey
In both men and women, a pattern was observed of initial increase in adjusted CKD stages 3–4 prevalence through the early 2000’s followed by stabilization through the end of the study period (p for sex-year interaction =0.61)(Figure 1B).
Adjusted prevalence of stage 3–4 CKD among non-Hispanic blacks continued to rise throughout the study period (Figure 1C). This was not the estimate for other race/ethnic groups. However, there was not a statistically significant difference in the trends by race/ethnicity (p-value for interaction =0.40).
Figure 1D shows the temporal trend in prevalence by diabetes status, adjusting for age, sex, and race/ethnicity. The initial increase in adjusted prevalence of CKD stages 3–4 stopped around the 2000’s among those classified as not having diabetes, while the prevalence appeared to continue to rise for those classified as having diabetes (although no statistically significant interaction was noted with p for diabetes-year interaction =0.23).
Expanded definition of CKD
Using the expanded definition of CKD, there was little change in crude CKD prevalence overall from 14.0% (12.4–15.5%) in 2003–04 to 14.2% (12.4–15.9%) in 2011–12 (Table 3)(Supplemental Table 3 shows number of NHANES participants providing data for each of the cells).
Table 3. Prevalence (percentage) of CKD (by CKD-EPI equation) with expanded definition which includes albuminuria ≥30 mg/g regardless of eGFR level by demographic and diabetic categories, NHANES 1988–1994 through 2011–2012;
95% confidence interval of prevalence (as percentage) are shown in parentheses; N represents the population number in 100,000’s
| Years | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1988–1994 | 1999–2000 | 2001–2002 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | |
|
| ||||||||
| Total Population
|
11.8% (10.9–12.7%) N = 198.1 |
13.2% (11.7–14.7%) N = 237 |
13.6% (12.4–14.8%) N = 256 |
14.0% (12.4–15.5%) N = 271 |
14.0% (11.9–16.2%) N = 277 |
14.1% (12.8–15.5%) N = 285.7 |
12.7% (11.5–13.9%) N = 261 |
14.2% (12.4–15.9%) N = 297 |
| Age | ||||||||
|
|
||||||||
| 20–39 years | 4.9% (4.1–5.7%) | 6.4% (3.9–8.9%) | 5.1% (3.5–6.6%) | 5.1% (3.6–6.7%) | 6.0% (5.2–6.9%) | 6.0% (4.8–7.2%) | 4.7% (3.8–5.7%) | 6.1% (4.6–7.6%) |
|
|
||||||||
| 40–64 years | 9.5% (8.3–10.7%) | 10.0% (8.2–11.9%) | 10.9% (9.3–12.4%) | 10.1% (8.3–11.9%) | 10.7% (8.1–13.3%) | 10.5% (8.6–12.3%) | 8.8% (7.5–10.0%) | 10.8% (8.6–13.1%) |
|
|
||||||||
| 65–79 years | 30.7% (27.5–33.9%) | 34.1% (30.0–38.2%) | 36.7% (32.6–40.8%) | 36.9% (31.6–42.3%) | 31.6% (28.4–34.7%) | 35.7% (33.7–37.7%) | 32.5% (28.8–36.2%) | 31.5% (28.9–34.2%) |
|
|
||||||||
| ≥80 years | 56.1% (52.1–60.0%) | 67.1% (59.3–74.9%) | 68.9% (62.4–75.4%) | 67.2% (63.2–71.1%) | 67.9% (62.1–73.7%) | 61.0% (51.3–70.7%) | 60.9% (55.4–66.5%) | 65.0% (58.2–71.8%) |
|
|
||||||||
| Sex | ||||||||
|
|
||||||||
| Male | 9.8% (8.4–11.1%) | 11.0% (9.4–12.6%) | 12.3% (11.0–13.7%) | 12.9% (11.2–14.6%) | 12.2% (9.4–15.0%) | 11.8% (10.5–13.0%) | 11.4% (10.2–12.6%) | 12.8% (10.2–15.4%) |
|
|
||||||||
| Female | 13.7% (12.4–15.0%) | 15.2% (13.3–17.0%) | 14.8% (13.1–16.5%) | 15.0% (13.2–16.7%) | 15.8% (13.2–18.4%) | 16.4% (14.3–18.5%) | 13.8% (12.1–15.5%) | 15.4% (13.6–17.2%) |
|
|
||||||||
| Race/ethnicity | ||||||||
|
|
||||||||
| Non-Hispanic white | 11.8% (10.6–13.1%) | 12.8% (11.2–14.4%) | 14.0% (12.8–15.2%) | 13.8% (12.2–15.3%) | 14.2% (11.7–16.7%) | 14.7% (13.1–16.3%) | 13.1% (11.7–14.5%) | 14.1% (11.9–16.3%) |
|
|
||||||||
| Non-Hispanic black | 13.7% (12.8–14.7%) | 14.2% (11.9–16.6%) | 14.9% (11.8–18.0%) | 14.4% (10.8–17.9%) | 15.6% (12.8–18.4%) | 15.3% (12.6–18.1%) | 14.0% (11.6–16.4%) | 17.3% (15.4–19.2%) |
|
|
||||||||
| Other race/ethnicity | 10.1% (8.0–12.2%) | 13.9% (10.1–17.7%) | 11.2% (8.8–13.6%) | 14.6% (11.3–17.8%) | 12.5% (9.6–15.3%) | 11.6% (9.6–13.5%) | 10.5% (8.3–12.6%) | 12.7% (10.0–15.5%) |
|
|
||||||||
| Diabetes status | ||||||||
|
|
||||||||
| With diabetes mellitus | 38.5% (35.1–41.9%) | 35.2% (30.3–40.2%) | 42.2% (37.0–47.3%) | 40.3% (35.6–45.0%) | 38.9% (32.4–45.3%) | 36.9% (33.7–40.2%) | 35.9% (31.0–40.9%) | 36.5% (32.2–40.8%) |
|
|
||||||||
| Without diabetes mellitus | 9.7% (8.9–10.4%) | 11.4% (9.8–12.9%) | 11.0% (9.6–12.5%) | 11.1% (9.2–12.9%) | 11.4% (9.3–13.6%) | 11.2% (9.8–12.6%) | 9.8% (8.8%–10.9%) | 11.3% (9.8–12.8%) |
During the study period, adjusting for age, sex, race/ethnicity and diabetes status, we observed a slight decrease in adjusted CKD prevalence overall (p =0.019)(red lines in Supplemental Figures 1A to 1D).
Using the expanded definition of CKD, consistent with the CKD definition based on eGFR only (Figure 1C), adjusted prevalence of disease among non-Hispanic blacks showed a continued increase through 2012, differing from the pattern in other race/ethnicity subgroups (although still no statistically significant interaction was noted with p for race/ethnicity-year interaction =0.24)( Supplemental Figure 1C). Among people with and without diabetes, using the expanded definition of CKD, there were no increases in the adjusted prevalence of disease (p for diabetes-year interaction =0.22) (Supplemental Figure 1D).
Sensitivity analysis using MDRD equation to estimate GFR
Largely similar results were seen in sensitivity analyses when the MDRD equation was used instead to define CKD (Supplemental Table 4 and 5 and Supplemental Figure 2 and 3).
CONCLUSIONS
In this study, we found encouraging news regarding the prevalence of CKD in the overall U.S. adult population. Our analyses indicate that, in a reversal of prior trends, there has been no appreciable increase in the prevalence of stages 3–4 CKD in the U.S. population overall during the most recent decade. This secular pattern is consistent with the more recent epidemiology of ESRD, which has also shown a stabilization of ESRD incidence in the United States since the early 2000’s (1). Our observations are also consistent with international studies, particularly a recent analysis of data from the nationally representative Health Survey for England random samples, which reported no increase in prevalence of CKD (defined there as eGFR <60 mL/min/1.73m2) from 2003 to 2009–10 (25).
Our findings based on more recent data are in contrast to prior peer-reviewed publications reporting analyses of older data (summarized in Supplemental Table 6). For example, Hsu et al. estimated that between NHANES II (1976–80) to NHANES III (1988–94), among those aged 20–74, overall prevalence of CKD (defined in that study as eGFR <60 mL/min/1.73m2) increased 1.7% per year (7). Coresh et al. reported a 3.5% yearly increase of stages 1–4 CKD from NHANES III (1988–94) to NHANES 1999–2004 using serum creatinine-based eGFR while Grams et al. reported a yearly increase of stages 3–4 CKD of up to 5.0% from NHANES III (1988–94) to NHANES 1999–2002 using cystatin C based eGFR (4, 5).
The lack of increase in CKD prevalence since the early 2000’s was a temporal trend also noted in the majority of subgroups examined, stratifying by age, sex, race/ethnicity as well as diabetes classification. However, for non-Hispanic blacks, the crude as well as adjusted CKD prevalence increased from the early 2000s through 2011–12. The increase in non-Hispanic blacks was consistently observed when CKD was defined either as eGFR of 15–59 mL/min/1.73m2 or with an expanded definition of CKD, which also counted persons with higher eGFR but a one-time urine ACR ≥30 mg/g. This increase in CKD prevalence in non-Hispanic blacks warrants careful monitoring. Prior studies have emphasized racial difference in progression of CKD towards ESRD (26) and there have been important recent advances in our understanding of the importance of genetic contributions in addition to well-known racial differences in clinical risk factors and disparities with regard to access to care (27). Further research will need to reconcile observations reported here with the encouraging secular pattern in ESRD in which the incidence of ESRD among blacks has fallen over time more notably than among whites in the United States (1). Specifically, the incidence of ESRD—although remaining at a much higher level in blacks--has dropped sooner and to a greater extent in blacks than in whites (age-sex adjusted incidence for blacks peaked in 2002 at 1078 per million/year and fell progressively to 865 per million/year in 2013 vs. highest observed incidence for whites was 299 per million/year in 2006 before falling to 286 per million/year in 2013)(1).
Our findings of CKD prevalence among those classified as having diabetes--especially the analysis taking into account the presence of albuminuria, a classic hallmark of diabetic kidney disease (Table 3 and Supplemental Figure 1D)--are consistent with a prior analysis of NHANES data through 2008 which reported no change in the prevalence of diabetic kidney disease (28). Our study also examined the concurrent prevalence of CKD among those not considered to have diabetes and extended the temporal trend analysis through 2012 to demonstrate continued stability.
Possible reasons for the recent stabilization of overall CKD prevalence--despite continued aging of the U.S. population and the increased prevalence of obesity--include successful adoption across the population of reno-protective measures discovered since the 1980s and considerable advances in medical treatments in the past several decades (29). For example, when the landmark United Kingdom Prospective Diabetes Study (UKPDS) trial was conducted in the 1980s, it was thought acceptable to allow patients with type 2 diabetes in the control arm to have blood pressures as high as 200/105 mmHg (this threshold was lowered to 180/105 mmHg in 1992)(30). The first large scale randomized control trial to demonstrate the reno-protective effect of renin-angiotensin system blockade was not published until 1993 (31). For patients with diabetes, large strides have also been made in glycemic control (32), which probably explains why a Finnish study found that those diagnosed with type 1 diabetes in 1980 through 1999 had less than half the risk of developing ESRD compared with those diagnosed in 1965 through 1969 (33). Reductions in the risk of ESRD among U.S. persons with type 2 diabetes have also been reported by the CDC (34). While the cross-sectional nature of NHANES data limits causal inference, data from serial NHANES surveys also show that hypertension control has improved over time (for example, reduced likelihood of uncontrolled blood pressure) among those with and without CKD (35–38). For example, from 1988–1994 to 1999–2000, the percent of US hypertensive adults who took a prescription antihypertensive medication increased from 57.3% to 62.9%, including a 68% increase in angiotensin-converting enzyme inhibitor use (36). From 2001–2002 to 2009–2010, the use of angiotensin-converting enzyme inhibitors increased by 31% and the use of angiotensin receptor blockers by 100%. Blood pressure control rates among hypertensive adults increased from 28.7% in 2001–2002 to 47.2% in 2009–2010. (Blood pressure control rates improved among those with diabetes mellitus from 35.6% to 44.6% and rates improved among those with chronic kidney disease from 29.6% to 43.7%)(37). There have also been clear improvements in glycemic control among patients with diabetes shown in serial NHANES analyses. For example, the prevalence of calibrated HbA1c levels less than 7.0% increased from 50.9% in 1988–1994 to 58.8% in 2005–2010 among adults with diagnosed diabetes. And among persons who reported currently taking medications for diabetes, the prevalence of calibrated HbA1c levels less than 7.0% increased from 39.7% in 1988–1994 to 55.1% in 2005–2010 (39). Additional strides have been made in treatment of less common causes of ESRD, such as multiple myeloma (40). Thus, it is very plausible that better medical management of these conditions has played a large role in halting the previous rising trend in CKD prevalence and which in turn resulted in the recent decline in the incident rate of ESRD.
Strengths of this study include use of rigorously-collected nationally representative survey data, which can generalize to the entire U.S. non-institutionalized adult population. NHANES collected data including a wide range of demographic information and comorbid conditions in a uniform manner throughout the study years and provided recommendations for the calibration of serum creatinine measurements. Our results are robust to alternative equations used to estimate GFR and whether or not we considered persons with elevated albuminuria.
In addition to the strengths, our study had several limitations. First, only one measurement of serum creatinine and urine albuminuria were available which may result in misclassification of CKD status. However, this should not bias our assessment of temporal trends since misclassification is likely to be non-differential with respect to calendar year. Another limitation is that we could only analyze temporal trends in CKD prevalence and not CKD incidence. Although conceivably an alternative explanation for our findings is an increase in mortality rates among persons with CKD in later calendar years, we are unaware of any data suggesting this is true. There is some misclassification of diabetes status since we used A1c and not fasting glucose levels or 2-h glucose challenge values to define diabetes (39, 41) and some patients with prediabetes may be prescribed metformin. Given the serial cross-sectional nature of the NHANES data, we are limited in our ability to assess the impact of secular trends in treatment, including differential rates of control of risk factors in demographic or disease subgroups. We did not analyze data on the impact of socio-economic status and correlated health behaviors and health care access.
CKD has been recognized as a major public health problem, associated with substantial morbidity, mortality as well as financial cost to the healthcare system. Information regarding the prevalence of CKD using nationally representative NHANES data has provided a benchmark for kidney disease studies, prevention efforts, and health care planning. Our finding that CKD prevalence has plateaued within the last decade is encouraging and important, but the apparent continued increase in CKD prevalence among non-Hispanic blacks is concerning. Reversal of the previously observed trend in increase in CKD prevalence in the overall population could potentially save lives and healthcare resources. Further research might be considered to explain observed trends in CKD prevalence and to better understand potentially important differences in patterns by race/ethnicity subgroups. Identification of particularly impactful strategies accounting for this would be important as ensuring more widespread adoption of such strategies could lead to large clinical and public health benefits and continue to sustain and improve CKD prevalence trends for all population groups.
Supplementary Material
Acknowledgments
We thank the participants and staff of the National Health and Nutrition Examination Survey.
Funding Sources: Centers for Disease Control and Prevention Cooperative Agreement (1U58DP003839); American Society of Nephrology Foundation for Kidney Research Student Scholar Grant Program; and National Institutes of Health (K24DK92291).
APPENDIX
The CDC CKD Surveillance Team consists of members groups led by the University of California, San Francisco (Neil Powe [Principal Investigator],* Tanushree Banerjee,* Chi-yuan Hsu,* Kirsten Bibbins-Domingo, Charles McCulloch,* Deidra Crews, Vanessa Grubbs, Delphine Tuot, Yunno Zhu), the University of Michigan (Rajiv Saran [Principal Investigator],* Yi Li, Jennifer Bragg-Gresham,* Vahakn Shahinian, Hal Morgenstern,* Michael Heung, Diane Steffick, Anca Tilea, Brenda Gillespie, William Herman, Jerry Yee, Kara Zivin, William McClellan, Deb Gipson, Sai Dharmarajan, Rajesh Balkrishnan, April Wyncott), and the CDC (Nilka Ríos Burrows [Technical Advisor], Desmond Williams, Mark Eberhardt,* Meda Pavkov,* Deborah Rolka, Sharon Saydah, Larry Waller).
*Meets criteria for authorship
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REPRODUCIBLE RESEARCH STATEMENT
Protocol: not available
Statistical Code: Available to approved persons through written agreement with the authors from Dr. Neil Powe (Neil.Powe@ucsf.edu) or Dr. Chi-yuan Hsu (hsuchi@medicine.ucsf.edu)
Data: available at http://www.cdc.gov/nchs/nhanes.htm
References
- 1. [accessed November 11, 2015];US Renal Data System 2015 Annual Data Report. at http://www.usrds.org/2015/view/Default.aspx.
- 2.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: Common, harmful, and treatable - World Kidney Day 2007. Journal of the American Society of Nephrology. 2007;18(2):374–8. doi: 10.1681/ASN.2006121305. [DOI] [PubMed] [Google Scholar]
- 3.El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331–40. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.Grams ME, Juraschek SP, Selvin E, Foster MC, Inker LA, Eckfeldt JH, et al. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. Am J Kidney Dis. 2013;62(2):253–60. doi: 10.1053/j.ajkd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu RK, Hsu CY. Temporal trends in prevalence of CKD: the glass is half full and not half empty. Am J Kidney Dis. 2013;62(2):214–6. doi: 10.1053/j.ajkd.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CY, Vittinghoff E, Lin F, Shlipak MG. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141(2):95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 8.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16(1):180–8. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Wang C, Snyder JJ, Collins AJ. Cystatin C levels in U.S. adults, 1988–1994 versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009;4(5):965–72. doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go A, Chertow G, Fan D, MuCulloch C, Hsu C. Chronic kidney disease and risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–7. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, et al. CKD prevalence varies across the European general population. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [accessed October 27, 2015];Healthy People 2020. at http://www.healthypeople.gov/2020/topics-objectives/topic/chronic-kidney-disease/objectives.
- 15.National Center for Health Statistics. [accessed November 12, 2015];National Health and Nutrition Examination Survey. at http://www.cdc.gov/nchs/nhanes.htm.
- 16.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 17.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Saydah SH, Pavkov ME, Zhang C, Lacher DA, Eberhardt MS, Burrows NR, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009–2010. Clin Chem. 2013;59(4):675–83. doi: 10.1373/clinchem.2012.195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. [accessed April 28, 2016];National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. at http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf. [PubMed]
- 23.National Center for Health Statistics. [accessed Decemeber 5, 2015];Analytic Guidelines. at http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf.
- 24.Vittinghoff EDG, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics. 2. Springer; 2012. Section 9.3.4. [Google Scholar]
- 25.Aitken GR, Roderick PJ, Fraser S, Mindell JS, O’Donoghue D, Day J, et al. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open. 2014;4(9):e005480. doi: 10.1136/bmjopen-2014-005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–7. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 27.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–96. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–9. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parving H-H, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet. 1983;1:1175–8. doi: 10.1016/s0140-6736(83)92462-5. [DOI] [PubMed] [Google Scholar]
- 30.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 32.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–76. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA. 2005;294(14):1782–7. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 34.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 35.Plantinga LC, Miller ER, 3rd, Stevens LA, Saran R, Messer K, Flowers N, et al. Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999–2006. Hypertension. 2009;54(1):47–56. doi: 10.1161/HYPERTENSIONAHA.109.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu QP, Paulose-Ram R, Dillon C, Burt V. Antihypertensive medication use among US adults with hypertension. Circulation. 2006;113(2):213–21. doi: 10.1161/CIRCULATIONAHA.105.542290. [DOI] [PubMed] [Google Scholar]
- 37.Gu QP, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension The National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–14. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SS, Gu QP, Nwankwo T, Wright JD, Hong YL, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015;65(1):54. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–25. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reule S, Sexton DJ, Solid CA, Chen S, Foley RN. ESRD due to multiple myeloma in the United States, 2001–2010. J Am Soc Nephrol. doi: 10.1681/asn.2014090876. Published online before print October 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–8. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.