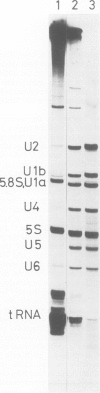
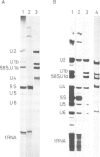
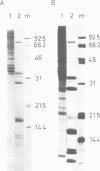
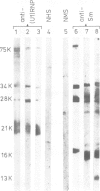
Abstract
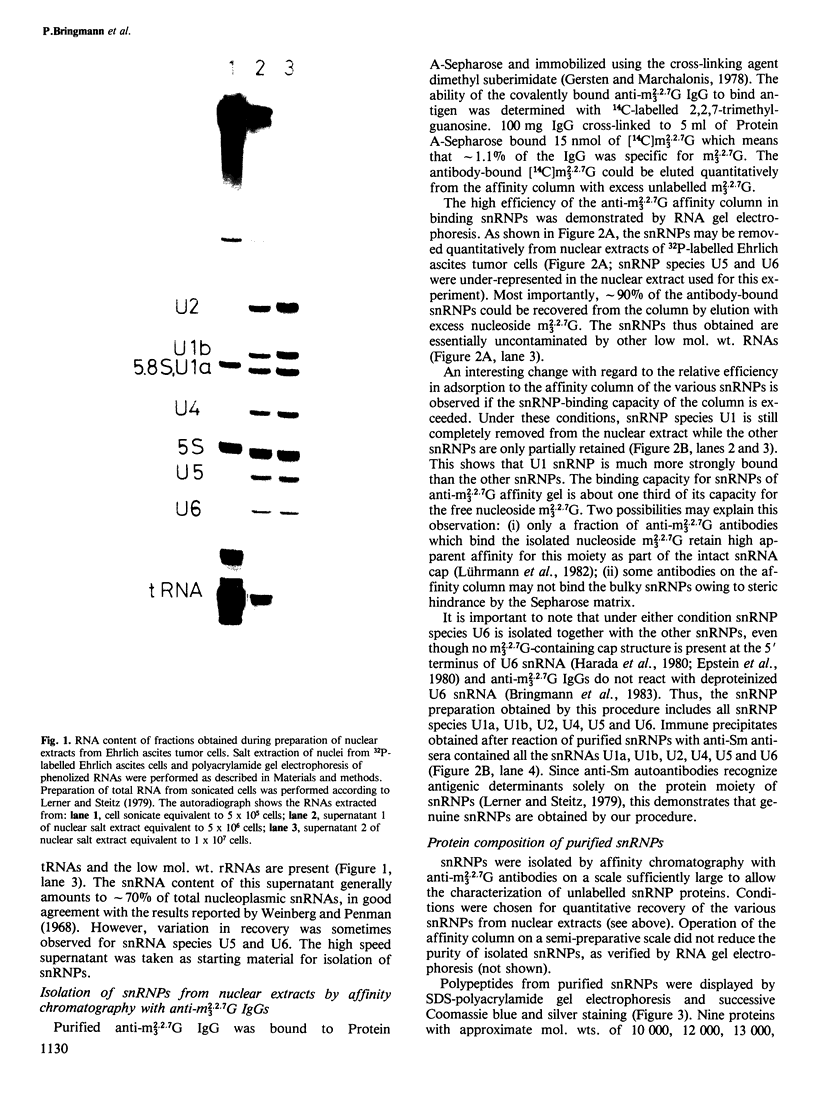
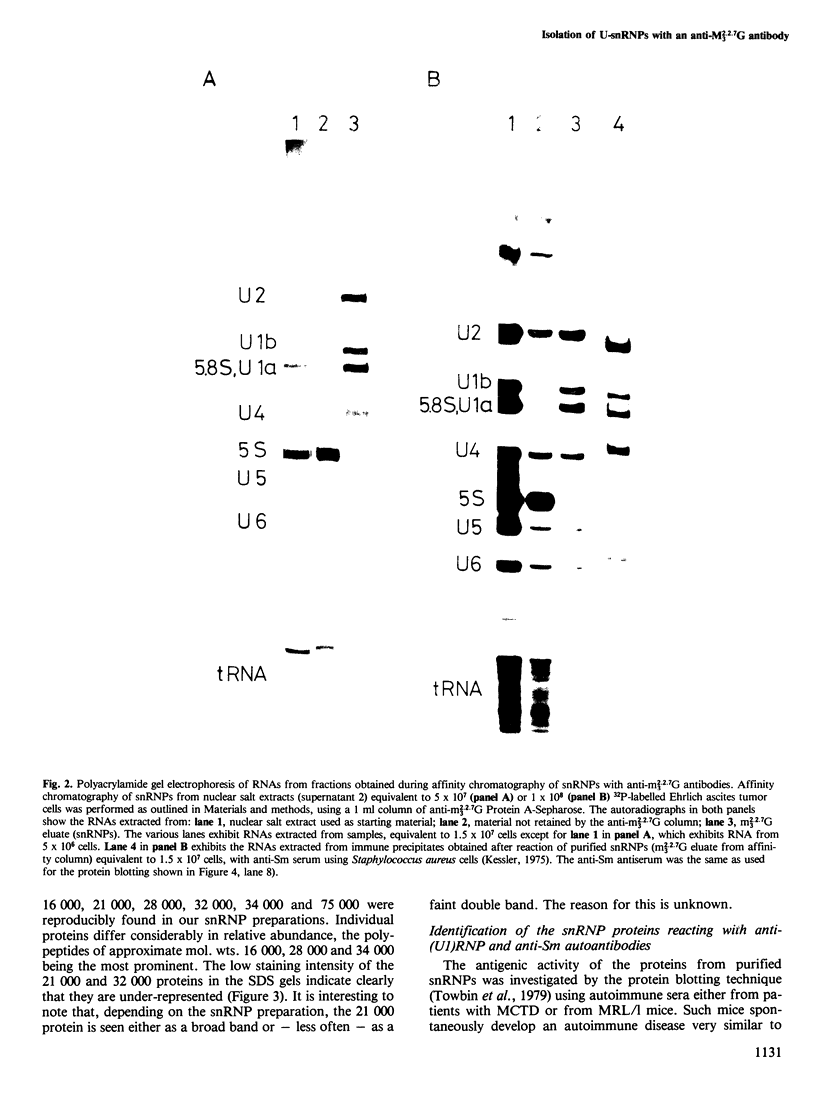
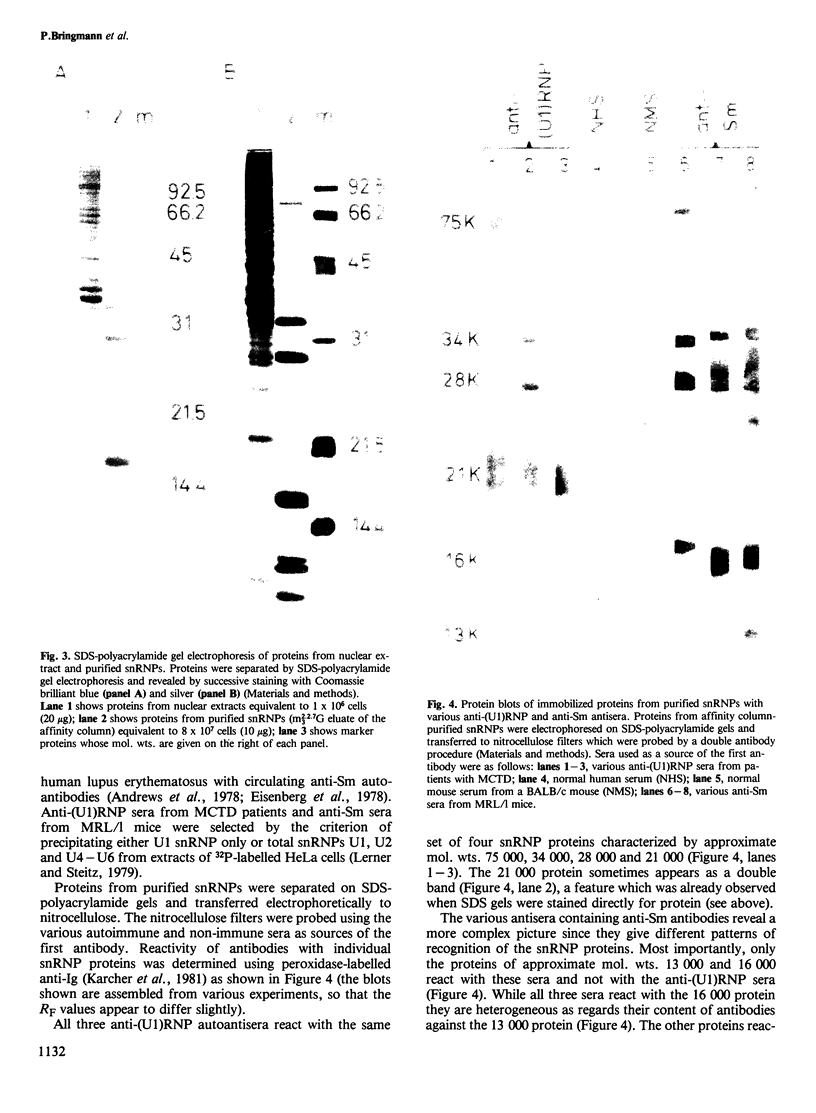
Small nuclear ribonucleoprotein particles (snRNPs) of the U-snRNP class from Ehrlich ascites tumor cells were purified in a one-step procedure by affinity chromatography with antibodies specific for 2,2,7-trimethylguanosine (m23.2.7G), which is part of the 5'-terminal cap structure of snRNAs U1-U5. Antibody-bound snRNPs are desorbed from the affinity column by elution with excess nucleoside m23.2.7G; this guarantees maintenance of their native structure. The snRNPs U1, U2, U4, U5 and U6 can be recovered quantitatively from nuclear extracts by this procedure. Co-isolation of U6 snRNP must be due to interactions between this and other snRNPs, as anti-m23.2.7G antibodies do not react with deproteinized U6 snRNA. We have so far defined nine proteins of approximate mol. wts. 10 000, 12 000, 13 000, 16 000, 21 000, 28 000, 32 000, 34 000 and 75 000. Purified snRNPs react with anti-(U1)RNP and with anti-Sm antisera from patients with mixed connective tissue disease and from MRL/l mice. As determined by the protein blotting technique, six of the snRNP polypeptides, characterized by apparent mol. wts. 13 000, 16 000, 21 000, 28 000, 34 000 and 75 000, bear antigenic determinants for one or the other of the above autoantibody classes. This suggests strongly that the U-snRNPs produced by the procedure described here are indeed representative of the snRNPs in the cell. With highly purified snRNPs available, investigation of possible enzymic functions of the particles may now be undertaken.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings P. B., Allen R. W., Jensen F. C., Hoch S. O. Anti-RNP monoclonal antibodies derived from a mouse strain with lupus-like autoimmunity. J Immunol. 1982 Mar;128(3):1176–1180. [PubMed] [Google Scholar]
- Bringmann P., Reuter R., Rinke J., Appel B., Bald R., Lührmann R. 5'-terminal caps of snRNAs are accessible for reaction with 2,2,7-trimethylguanosine-specific antibody in intact snRNPs. J Biol Chem. 1983 Mar 10;258(5):2745–2747. [PubMed] [Google Scholar]
- Brunel C., Widada J. S., Lelay M. N., Jeanteur P., Liautard J. P. Purification and characterization of a simple ribonucleoprotein particle containing small nucleoplasmic RNAs (snRNP) as a subset of RNP containing heterogenous nuclear RNA (hnRNP) from HeLa cells. Nucleic Acids Res. 1981 Feb 25;9(4):815–830. doi: 10.1093/nar/9.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Meyer L. M., Pederson T. Small nuclear RNA U2 is base-paired to heterogeneous nuclear RNA. Science. 1982 Jul 30;217(4558):456–458. doi: 10.1126/science.6178162. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Base-pairing interactions between small nuclear RNAs and nuclear RNA precursors as revealed by psoralen cross-linking in vivo. Cell. 1981 Nov;26(3 Pt 1):363–370. doi: 10.1016/0092-8674(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Conner G. E., Nelson D., Wisniewolski R., Lahita R. G., Blobel G., Kunkel H. G. Protein antigens of the RNA-protein complexes detected by anti-SM and anti-RNP antibodies found in serum of patients with systemic lupus erythematosus and related disorders. J Exp Med. 1982 Nov 1;156(5):1475–1485. doi: 10.1084/jem.156.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. A., Tan E. M., Dixon F. J. Presence of anti-Sm reactivity in autoimmune mouse strains. J Exp Med. 1978 Feb 1;147(2):582–587. doi: 10.1084/jem.147.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Henning D., Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980 Sep 25;255(18):8901–8906. [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Gersten D. M., Marchalonis J. J. A rapid, novel method for the solid-phase derivatization of IgG antibodies for immune-affinity chromatography. J Immunol Methods. 1978;24(3-4):305–309. doi: 10.1016/0022-1759(78)90133-3. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- Karcher D., Lowenthal A., Thormar H., Noppe M. Serological identification of viral antigens after electrophoretic transfer. J Immunol Methods. 1981;43(2):175–179. doi: 10.1016/0022-1759(81)90021-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrmann R., Appel B., Bringmann P., Rinke J., Reuter R., Rothe S., Bald R. Isolation and characterization of rabbit anti-m3 2,2,7G antibodies. Nucleic Acids Res. 1982 Nov 25;10(22):7103–7113. doi: 10.1093/nar/10.22.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray A. J., Carroll A. R., Dahi S., Naxakis G., Sadaie M. R., Wallis C. M., Jing T. The composition of the nuclear antigens Sm and RNP of human rheumatic and connective tissue diseases and the relevance of their autoantibodies as probes for RNA processing mechanisms. FEBS Lett. 1982 May 17;141(2):139–147. doi: 10.1016/0014-5793(82)80033-1. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Ro-Choi T. S., Busch H. Nuclear ribonucleoprotein complexes containing U1 and U2 RNA. Biochemistry. 1975 Oct 7;14(20):4380–4385. doi: 10.1021/bi00691a006. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeris C. E., Niessing J. Evidence for the existence of a structural RNA component in the nuclear ribonucleoprotein particles containing heterogeneous RNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):642–650. doi: 10.1016/0006-291x(75)90447-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R., Penman S. Metabolism of small molecular weight monodisperse nuclear RNA. Biochim Biophys Acta. 1969 Sep 17;190(1):10–29. doi: 10.1016/0005-2787(69)90150-6. [DOI] [PubMed] [Google Scholar]
- White P. J., Hoch S. O. Definition of the antigenic polypeptides in the Sm and RNP ribonucleoprotein complexes. Biochem Biophys Res Commun. 1981 Sep 16;102(1):365–371. doi: 10.1016/0006-291x(81)91530-8. [DOI] [PubMed] [Google Scholar]
- Zieve G., Penman S. Subnuclear particles containing a small nuclear RNA and heterogeneous nuclear RNA. J Mol Biol. 1981 Jan 25;145(3):501–523. doi: 10.1016/0022-2836(81)90542-8. [DOI] [PubMed] [Google Scholar]