Abstract
Objective
To prospectively evaluate the safety and therapeutic effectiveness of dual-switching monopolar (DSM) radiofrequency ablation (RFA) for the treatment of hepatocellular carcinoma (HCC), and to retrospectively compare the results with those of single-switching monopolar (SSM) RFA in a historical control group.
Materials and Methods
This study was approved by the Institutional Review Board, with informed consent obtained from all patients. Fifty-two HCC patients who underwent DSM-RFA using a separable clustered electrode and dual-generators were prospectively enrolled. Technical parameters, complications, technical success, technical effectiveness, and local tumor progression (LTP) rates were evaluated by means of post-procedural and follow-up imaging. Thereafter, the outcome of DSM-RFA was compared with those of 249 retrospectively included HCC patients treated with SSM-RFA.
Results
There were two major complications (3.8%, 2/52) including pleural and pericardial effusion in the DSM-RFA group. The DSM-RFA yielded a 100% technical success rate, a 98.1% technical effectiveness rate, and a 4.3% 2-year LTP rate. In a retrospective comparison between the two groups, DSM-RFA created significantly larger ablation volume (4.20 ± 2.07 cm3/min vs. 3.03 ± 1.99 cm3/min, p < 0.01), and delivered higher energy (1.43 ± 0.37 kcal/min vs. 1.25 ± 0.50 kcal/min, p < 0.01) per given time, than SSM-RFA. There was no significant difference in major procedure-related complications (3.8% vs. 4.4%) and technical effectiveness rate (98.1% vs. 96.4%) between the two groups (p = 1.00). In addition, the 2-year LTP rate of DSM-RFA and SSM-RFA were 4.3% and 10.1%, respectively (p = 0.15).
Conclusion
DSM-RFA using a separable clustered electrode is safe and provides high local tumor control and good preliminary clinical outcome for small HCCs, which are at least comparable to those of SSM-RFA.
Keywords: Liver, Hepatocellular carcinoma, Local ablation therapy, Radiofrequency ablation, Therapeutic efficacy
INTRODUCTION
Percutaneous image-guided radiofrequency ablation (RFA) has been widely accepted as a promising, noninvasive treatment option for early stage hepatocellular carcinoma (HCC) and a potential alternative to surgical resection with a higher cost effectiveness for small HCCs < 3 cm in diameter (1,2,3,4). However, the higher local tumor progression (LTP) rate of RFA compared to that of surgery remains one of its major limitations (5,6). To achieve the best LTP rate in patients with HCC after RFA, the creation of a safety margin 5–10 mm around the target tumor is generally recommended (7,8,9). Yet, for most RFA systems and electrodes clinically available today, the diameter of the ablation zone able to be induced by a single ablation is limited to 3–4 cm (10). Therefore, particularly for tumors > 2 cm, multiple electrode repositionings are often required to achieve complete three-dimensional ablation of the tumor. However, since most percutaneous RFA procedures are performed under ultrasound guidance, especially in Asia, repositioning the electrodes accurately in the remaining viable tumor portion remains technically challenging owing to gas bubble shadows formed in the ablated tissue (1,11). Indeed, according to a recent paper by Kim et al. (12) on the 10-year outcomes of percutaneous RFA for early HCCs, the cumulative LTP rates were 27.0% and 36.9% at 5 years and 10 years, respectively. Furthermore, although no significant differences in survival have been demonstrated between patients with and without LTP during the 5 to 10 year follow-up period, it can be expected that the development of LTP would significantly shorten median recurrence-free survival, necessitating a higher number of interventional procedures. Therefore, development of more potent RFA equipment with better efficiency in creating larger ablation volumes would be of great clinical value helping to lower LTP rates after RFA. To overcome this limitation of conventional monopolar RFA, investigators have begun to perform RFA using multiple electrodes with a switching system allowing the operator to switch between monopolar, bipolar or multipolar modes (13,14,15,16,17,18). According to these studies, the multiple electrode approach was able to induce a larger ablation zone compared to conventional monopolar RFA using a single electrode with reported three year LTP rates of 11.0–23.8% (13,14,15,16,17,18). More recently, to further increase the efficiency of RF energy delivery, a dual-switching monopolar (DSM) RFA system using separable clustered electrodes and a three-channel dual-generator was developed. This allows RF energy to be applied to two electrodes simultaneously (19,20). However, to the best of our knowledge, there have been no previous studies reporting the clinical experience of DSM-RFA in patients with HCC.
Therefore, the purpose of our study was to prospectively evaluate the safety and therapeutic effectiveness of DSM-RFA for the treatment of HCC, and to retrospectively compare the results with those of single-switching monopolar (SSM)-RFA in a historical control group.
MATERIALS AND METHODS
The Institutional Review Board of our institution approved this prospective study of DSM-RFA and also a retrospective comparison of preliminary clinical outcomes between the DSM-RFA group and the historical control group of SSM-RFA. All patients who underwent DSM-RFA gave written informed consent prior to the RFA procedure. Financial support was provided by STARmed Co., Ltd. (Goyang, Korea). The authors had complete control of the data in this study, which was unbiased by industry.
Patients
In our institute, the general indications for RFA in HCC patients were as follows: 1) a single nodular HCC less than 5 cm in diameter, but preferentially less than 3 cm, or 2) two or three HCCs less than 3 cm; and 3) clinically significant portal hypertension and liver cirrhosis. However, hepatic resection candidates who preferred RFA over hepatic resection, in spite of the strong recommendation for hepatic resection by the clinicians, are also regarded as an indication of RFA. For this study, the indications for DSM-RFA and SSM-RFA were identical. Among the patients scheduled for RFA from July 2013 to June 2014, patients who met the inclusion and exclusion criteria and gave the informed consent were enrolled for DSM-RFA. The inclusion criteria were as follows: 1) patients whose ages ranged from 20 to 75 years old, 2) patients with liver cirrhosis and scheduled to undergo RFA for HCC visualized on CT or MRI performed within 30 days before the procedure, and 3) no previous locoregional treatment for index tumors including RFA, percutaneous ethanol injection, transarterial chemoembolization (TACE), or surgical resection. Among them, we excluded the following patients: 1) patients with three or more hepatic lesions, 2) tumors with the largest diameter > 5 cm, 3) tumors abutting the central portal vein or hepatic vein with a diameter > 5 mm, 4) compromised hepatic function of Child-Pugh class C, 5) overt tumor thrombus in the portal or hepatic vein on CT or MRI, and 6) platelet count < 50000 per µL or international normalized ratio (INR) > 1.5, or (g) overt extrahepatic metastasis.
Hepatocellular carcinoma was diagnosed either by pathology or by imaging modalities according to the American Association for the Study of Liver Diseases practice guidelines (3) or the Liver Imaging-Reporting and Data System if gadoxetic acid-enhanced MRI was used (21,22). Finally, 52 HCC patients (M:F = 39:13; mean age, 59 years; age range, 31–74 years) comprised our study population.
Radiofrequency Ablation Procedure
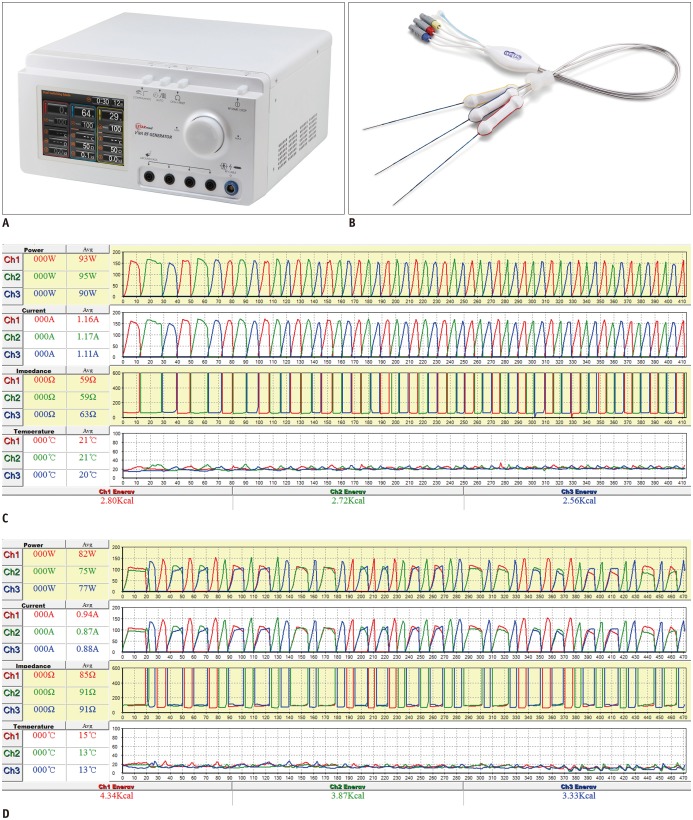
All RFA procedures were performed on an inpatient basis by one of three attending radiologists (with 17, 8, and 10 years of experience in RFA, respectively). Conscious sedation was induced and patient's vital signs were continuously monitored during the procedure. RFA was performed using a separable clustered electrode (Octopus®, STARmed, Goyang, Korea) and a three-channel dual-generator (VIVA Multi®, STARmed) under the guidance of real-time fusion imaging (PercuNav, Philips Healthcare, Best, the Netherlands; Virtual Navigator, Esaote, Genoa-Firenze, Italy) (Fig. 1) (23,24). The separable clustered electrode was composed of three internally cooled electrodes. Unlike conventional clustered electrodes, each individual electrode was separable and could be inserted either as a group at a fixed distance of 5 mm, or as three single electrodes with variable inter-electrode distances (16). This three-channel RF system with two independent 200 W generators allowed both the SSM and DSM modes. The DSM mode of the three channel dual-generator RF system was approved for clinical use by the Korean Ministry of Food and Drug Safety, and has been commercially available in Korea. In the SSM mode, RF energy (maximum: 200 W) was delivered to one of three electrodes and was automatically switched to another electrode every 30 seconds. However, if there was an increase in impedance value of at least 50 Ω above baseline, RF energy was immediately automatically switched to the other electrode. If tissue impedance was increased to 300 Ω above baseline, energy was not delivered to that particular electrode for 15 seconds. In the DSM mode, synchronous parallel RF energy was delivered to two of the three electrodes. Similar to the SSM mode, the current was automatically switched to a different combination of a pair of electrodes every 30 seconds. However, if impedance of either one of the electrodes rose above 170% of the baseline value at any time, the power was switched to form another pair of electrodes. For DSM-RFA, initially, the maximum RF energy delivered was limited to 240 W (120 + 120 W) for one minute and then increased up to 330 W (165 + 165 W) until tissue impedance rose to 170% of baseline values. Thereafter, maximum RF energy up to 200 W for each electrode was alternatively delivered to one or a pair of electrodes to prevent overly rapid impedance rises as demonstrated in previous ex vivo and in vivo animal studies (Fig. 1) (19,20). In addition, to prevent tract seeding, tract ablation was performed while maintaining the electrode tip temperature at 90℃ during withdrawal of the electrodes. For the ablation of peripheral tumors located near abdominal walls, diaphragms, or the bowel, or for tumors located in the hepatic dome portion, a 5% dextrose solution was instilled to establish artificial ascites and to isolate the liver or to improve the sonic window depending on the operator's need (1,25).
Fig. 1. Radiofrequency ablation devices and diagrams of radiofrequency energy delivery patterns during SSM- and DSM-RFA.
Photograph of three-channel dual-generator (VIVA Multi®) (A) and separable clustered electrode (Octopus®) (B), and records of applied current, power output, and impedance during SSM-RFA (C) and DSM-RFA (D). C. In SSM mode, RF energy was delivered to one of three electrodes and was automatically switched to another electrode based on impedance values. D. In DSM mode, RF energy was alternatively delivered to one or pair of electrodes based on tissue impedance changes, similar to SSM-RFA. DSM = dual-switching monopolar, RFA = radiofrequency ablation, SSM = single-switching monopolar
Post-Treatment Assessment and Follow-Up
Immediately after the RFA procedure, the patients underwent contrast-enhanced CT examinations including pre-contrast, arterial, and portal venous phases. These immediate post-RFA CT images were carefully reviewed by the operator, to assess technical success and procedure-related complications. Ablations were considered to be technically successful when the low attenuation ablation zone on the portal phase image included the entire tumor volume and, ideally, a tumor-free margin ≥ 5 mm (26). If ablation was considered to be insufficient, additional ablation was performed during the same hospital stay.
To assess the ablation volume, one author reviewed the immediate post-RFA CT images and measured the maximum (Dmx) and minimum (Dmi) diameters on the axial image with the largest ablation area, as well as the vertical diameter (Dv) of the ablation zone. The volumes of the ablation zone and the effectively ablated volumes were calculated by approximating the ablation zone to an ellipsoid using the following formula (14):
Follow-up CT or MRI was performed at 1 month and every 3 months following the ablation procedure. Two radiologists (with 7 and 3 years of experience in abdominal imaging, respectively) reviewed follow-up images, and evaluated technical effectiveness and LTP in consensus manner. Technical effectiveness was defined as when the ablation zone was larger than the ablated tumor and no enhancement was observed in and around the ablation zone on follow-up CT or MR images obtained immediately and one month after the ablation (26,27). LTP was defined as a newly appeared solid, arterial enhancing lesion at the margin of the ablated tumor at which technical effectiveness was reported on one month follow-up imaging according to the reporting criteria suggested by the International Working Group on Image-guided Tumor Ablation (27).
Complications
Patients were closely observed for possible complications during the ablation procedure and hospital stay. Complications were reported and categorized according to the Society of Interventional Radiology standards (27,28). A major complication was defined as an event that leads to substantial morbidity which increases the level of care or requires additional intervention, or substantially lengthens the hospital stay. All other complications were considered minor. A side effect was defined as an expected, commonly found, undesired consequence of the procedure which rarely causes substantial morbidity, such as abdominal pain, or asymptomatic pleural effusion. As these side effects do not increase the level of care unexpectedly, they are not considered true complications (27).
Comparison of the DSM-RFA Group with a Historical Control Group of SSM-RFA
HCC patients who underwent SSM-RFA between January 2011 and October 2014. As a result, 552 patients with 648 HCCs who were treated with SSM-RFA using the same separable clustered electrodes used for DSM-RFA were found. Among them, we only included 249 patients with 269 HCCs who met the same inclusion and exclusion criteria applied to the DSM-RFA group, to make indications for RFA similar in both groups. Finally, 249 patients (M:F = 188:61; mean age, 62 years; age range, 33–86 years) comprised a historical control group of SSM-RFA in our study. Technical parameters and clinical outcomes were compared between DSM-RFA and SSM-RFA groups to evaluate the efficacy of DSM-RFA.
Statistical Analysis
Data were compared between the DSM-RFA and SSM-RFA groups using the Fisher's exact test for categorical variables and using the independent t test for continuous variables. Technical parameters including diameter and volume of the ablation zone, delivered RF energy, and ablation time were compared on a per-nodule basis, whereas technical success, technical effectiveness, and LTP rates were compared on a per-patient basis. LTP rates were calculated using the Kaplan-Meier method, and subgroup comparisons were performed using the log-rank test. As the Kaplan-Meier analysis was performed on a per-patient basis, if LTP was identified at one of the two treated HCCs in a patient with two HCCs, the follow-up data of the patient were censored on the date of LTP. A p value < 0.05 was considered to indicate a statistically significant difference. A statistical software program (SPSS; version 19.0, SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
RESULTS
Baseline Characteristics
The patients' baseline characteristics are presented in Table 1. DSM-RFA group showed slightly higher serum albumin level (4.05 ± 0.38 vs. 3.82 ± 0.49, p = 0.01) and INR (1.12 ± 0.12 vs. 1.09 ± 0.11, p = 0.05) compared to SSM-RFA group. However, there was no significant difference in other baseline characteristics including age, sex, size and number of the tumor, subcapsular location of the tumor, serum bilirubin level, platelet count, alpha-fetoprotein (AFP) level, and Child-Pugh class.
Table 1. Patient Characteristics.
| Characteristics | SSM-RFA (n = 249*) | DSM-RFA (n = 52*) | P† |
|---|---|---|---|
| Age (mean ± SD) | 61.9 ± 9.5 | 59.1 ± 9.2 | 0.05 |
| Male, % | 75.5 (188/249) | 75.0 (39/52) | 1.00 |
| Single HCC, % | 92.0 (229/249) | 90.4 (47/52) | 0.79 |
| Size, cm (mean ± SD)‡ | 1.66 ± 0.63 | 1.54 ± 0.56 | 0.18 |
| Subcapsular location‡, % | 45.4 (122/269) | 59.6 (34/57) | 0.06 |
| AFP, ng/mL (mean ± SD) | 105.0 ± 686.2 | 117.9 ± 354.7 | 0.90 |
| AFP > 400 ng/mL, % | 3.2 (8/249) | 9.6 (5/52) | 0.05 |
| Child-Pugh class, % | 0.55 | ||
| A | 92.4 (230/249) | 96.2 (50/52) | |
| B | 7.6 (19/249) | 3.8 (2/52) | |
| Albumin, g/dL (mean ± SD) | 3.82 ± 0.49 | 4.05 ± 0.38 | 0.01 |
| Bilirubin, mg/dL (mean ± SD) | 0.97 ± 0.50 | 1.02 ± 0.65 | 0.58 |
| INR (mean ± SD) | 1.09 ± 0.11 | 1.12 ± 0.12 | 0.05 |
| Platelet, × 1000/mm3 (mean ± SD) | 121.9 ± 51.4 | 117.5 ± 57.2 | 0.59 |
*Numbers in parentheses represent number of patients, †Data were evaluated using Fisher's exact test for categorical variables and independent t test for continuous variables, ‡Size and frequency of subcapsular tumor were compared on per-nodule basis between SSM-RFA (269 HCCs) and DSM-RFA (57 HCCs). AFP = alpha-fetoprotein, DSM = dual-switching monopolar, HCC = hepatocellular carcinoma, INR = international normalized ratio, RFA = radiofrequency ablation, SD = standard deviation, SSM = single-switching monopolar
Artificial Ascites
Artificial ascites was induced in 44.2% (23/52) of the patients treated with DSM-RFA, and in 32.5% (81/249) of those who underwent SSM-RFA (p = 0.11). The amount of 5% dextrose solution instilled to establish artificial ascites was 572.2 ± 282.1 mL in the DSM-RFA group and 529.6 ± 275.5 mL in the SSM-RFA group, respectively (p = 0.52).
Complications and Side Effects
There was no patient who expired within 30 days after the ablation using either technique. In terms of major procedure-related complications, there was no significant difference between the two groups (3.8% [2/52] vs. 4.4% [11/249], p = 1.00). Major complications that occurred in patients who underwent DSM-RFA included pericardial effusion (which was under close observation at intensive care unit, but resolved spontaneously without additional intervention [n = 1]), and pleural effusion which required thoracentesis (n = 1). Major complications that occurred in the SSM-RFA group were as follows: active bleeding from the intercostal artery requiring transcatheter arterial embolization (n = 2), abscess formation requiring percutaneous drainage tube insertion (n = 1), pleural effusion requiring thoracentesis (n = 2), and sustained high fever requiring extended hospital stay (7 to 9 days after the procedure) and medical management (n = 6). In addition, there was one patient who developed a minor complication after the SSM-RFA procedure: a small amount of pneumothorax detected on immediate follow-up CT, which spontaneously resolved without chest tube insertion (n = 1).
Asymptomatic pleural effusion which did not require thoracentesis was noted in 23.1% (12/52) of the DSM-RFA group, and 19.7% (49/249) of the SSM-RFA group, respectively (p = 0.57). The artificial ascites technique had been used during the RFA procedure in 83.6% (51/61) of the 61 patients who developed post-procedural asymptomatic pleural effusion. In addition, 49.0% (51/104) of the patients who underwent RFA using the artificial ascites technique developed asymptomatic pleural effusion. Minimal collateral damage on post-procedural CT which did not cause clinical symptoms, require additional intervention, or lengthen the hospital stay were found in 9.6% (5/52) of the DSM-RFA group, and 8.4% (21/249) of the SSM-RFA group (p = 0.79).
Technical Parameters of the RFA Procedure
Among the 25 patients with two HCCs, five were excluded from technical parameter analysis because the ablation zone of the two lesions overlapped and could not be evaluated separately. As a result, technical parameters of 57 HCCs in DSM-RFA group were compared to those of 259 HCCs treated with SSM-RFA.
Compared to the SSM-RFA group, the DSM-RFA group showed significantly larger ablation volume (48.27 ± 28.55 cm3 vs. 38.08 ± 23.30 cm3, p = 0.004), larger ablation volume per given time (4.20 ± 2.07 cm3/min vs. 3.03 ± 1.99 cm3/min, p < 0.001), larger effectively ablated volume (32.21 ± 24.30 cm3 vs. 23.86 ± 18.33 cm3, p = 0.017), larger effectively ablated volume per given time (2.76 ± 1.60 cm3/min vs. 1.93 ± 1.68 cm3/min, p = 0.001), and higher delivered energy per given time (1.43 ± 0.37 kcal/min vs. 1.25 ± 0.50 kcal/min, p = 0.003). There was no significant difference in ablation time, and delivered energy between the two groups (p > 0.05) (Table 2).
Table 2. Comparison of Technical Parameters and Measured Size of Ablation Zones in DSM-RFA and SSM-RFA Groups.
| Variable | Total | Single HCC | ||||
|---|---|---|---|---|---|---|
| SSM-RFA | DSM-RFA | P* | SSM-RFA | DSM-RFA | P* | |
| No. of HCC | 259 | 57 | 229 | 47 | ||
| Dmx (cm) | 4.48 ± 1.02 | 5.04 ± 1.11 | < 0.001 | 4.58 ± 1.01 | 5.13 ± 1.11 | 0.01 |
| Dmi (cm) | 3.38 ± 0.82 | 3.76 ± 0.85 | 0.002 | 3.45 ± 0.81 | 3.79 ± 0.88 | 0.01 |
| Dv (cm) | 4.39 ± 1.24 | 4.56 ± 1.14 | 0.331 | 4.45 ± 1.22 | 4.56 ± 1.16 | 0.59 |
| Dmi/Dmx ratio | 0.76 ± 0.14 | 0.76 ± 0.14 | 0.874 | 0.77 ± 0.14 | 0.75 ± 0.14 | 0.54 |
| Ablation volume (cm3) | 38.08 ± 23.30 | 48.27 ± 28.55 | 0.004 | 40.09 ± 23.58 | 49.58 ± 29.44 | 0.04 |
| Ablation volume/time (cm3/min) | 3.03 ± 1.99 | 4.20 ± 2.07 | < 0.001 | 3.16 ± 2.03 | 4.04 ± 2.04 | 0.02 |
| Effectively ablated volume (cm3) | 23.86 ± 18.33 | 32.21 ± 24.30 | 0.017 | 25.15 ± 18.58 | 33.35 ± 25.97 | 0.02 |
| Effectively ablated volume/time (cm3/min) | 1.93 ± 1.68 | 2.76 ± 1.60 | 0.001 | 2.02 ± 1.72 | 2.63 ± 1.47 | 0.01 |
| Energy (kcal) | 16.79 ± 9.84 | 17.75 ± 10.43 | 0.510 | 17.37 ± 10.11 | 18.84 ± 11.02 | 0.37 |
| Energy/time (kcal/min) | 1.25 ± 0.50 | 1.43 ± 0.37 | 0.003 | 1.27 ± 0.47 | 1.41 ± 0.40 | 0.04 |
| Ablation time (min) | 13.70 ± 5.90 | 12.35 ± 6.10 | 0.121 | 13.69 ± 5.58 | 13.13 ± 6.31 | 0.54 |
*Data were evaluated using independent t test. Dmi = minimum diameter of ablation zone, Dmx = maximum diameter of ablation zone, Dv = vertical diameter of ablation zone
Subgroup analysis performed in patients with single HCC showed similar results. Compared to the SSM-RFA group, the DSM-RFA group showed significantly larger ablation volume (49.58 ± 29.44 cm3 vs. 40.09 ± 23.58 cm3, p = 0.04), larger ablation volume per given time (4.04 ± 2.04 cm3/min vs. 3.16 ± 2.03 cm3/min, p = 0.02), larger effectively ablated volume (33.35 ± 25.97 cm3 vs. 25.15 ± 18.58 cm3, p = 0.02), larger effectively ablated volume per given time (2.63 ± 1.47 cm3/min vs. 2.02 ± 1.72 cm3/min, p = 0.01), and higher delivered energy per given time (1.41 ± 0.40 kcal/min vs. 1.27 ± 0.47 kcal/min, p = 0.04) (Table 2).
Technical Success, Technical Effectiveness, and Local Tumor Progression Rate
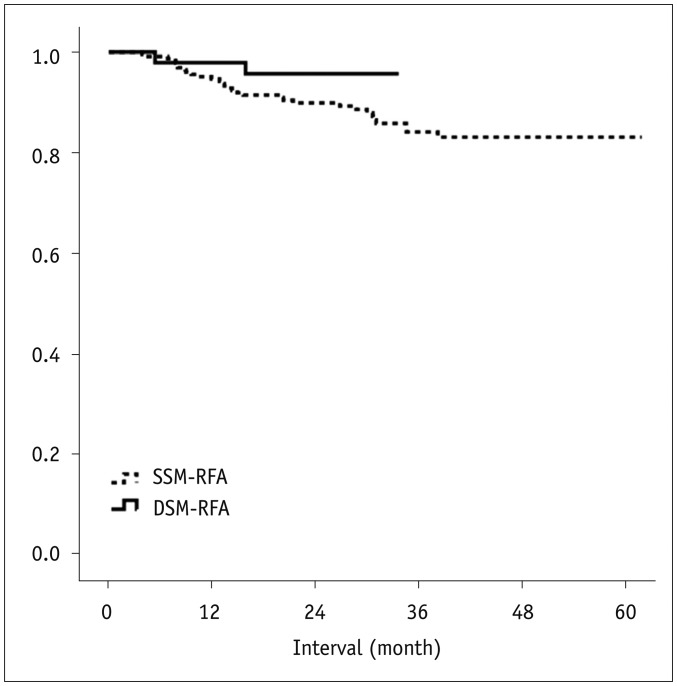
The technical success rates of the DSM-RFA and SSM-RFA were 100.0 (52/52) and 98.0% (244/249), respectively (p = 0.59). Technical effectiveness rates of the DSM-RFA and SSM-RFA were 98.1 (51/52) and 96.4% (240/249), respectively (p = 1.00) (Table 3). The one- and two-year LTP rates of the DSM-RFA vs. SSM-RFA were 2.1% (95% confidence interval [CI]: 0.3–13.9) vs. 5.4% (95% CI: 3.1–9.2) and 4.3% (95% CI: 1.1–16.2) vs. 10.1% (95% CI: 6.8–14.9), respectively (p = 0.15) (Table 3, Fig. 2).
Table 3. Technical Success, Technical Effectiveness, and Local Tumor Progression Rates in SSM-RFA and DSM-RFA Groups.
| Variable | SSM-RFA (n = 249*) | DSM-RFA (n = 52*) | P |
|---|---|---|---|
| Technical success rate, % | 98.0 (244/249) | 100.0 (52/52) | 0.59† |
| Technical effectiveness rate, % | 96.4 (240/249) | 98.1 (51/52) | 1.00† |
| Local tumor progression rate, % (95% CI) | 0.15‡ | ||
| 1 year | 5.4 (3.1–9.2) | 2.1 (0.3–13.9) | |
| 2 years | 10.1 (6.8–14.9) | 4.3 (1.1–16.2) |
*Number of patients, †Data were evaluated using Fisher's exact test, ‡p value was calculated using Kaplan-Meier method (log-rank test).
CI = confidence interval
Fig. 2. Life-table survival curve of local tumor progression-free survival in patients treated with DSM and SSM-RFA.
One- and two-year local tumor progression-free survival rates of DSM-RFA group vs. SSM-RFA group were 97.9% vs. 94.6% and 95.7% vs. 89.9%, respectively. p value was 0.149, which was calculated using log-rank test.
DISCUSSION
Our study demonstrated that DSM-RFA using the dual generator system and a separable clustered electrode provided clinical outcomes including a high technical success rate of 100.0% (52/52), a high technical effectiveness rate of 98.1% (51/52), and a low two-year LTP rate of 4.3%. In addition, our comparative analysis of the DSM-RFA and SSM-RFA demonstrated that DSM-RFA was capable of inducing larger ablation zones compared to SSM-RFA when treating HCCs (48.27 ± 28.55 cm3 vs. 38.08 ± 23.30 cm3, p = 0.004), while no significant differences were observed in terms of procedure-related complications, technical success rates, and technical effectiveness rates (p > 0.05). In addition, although no statistical difference was found, the one- and two-year LTP rates of the DSM-RFA (2.1% and 4.3%) in our study were lower than those of SSM-RFA (5.4% and 10.1%) in our historical comparison group. Therefore, based on our study results, we believe that the DSM-RFA is technically feasible, safe, and more effective in inducing larger ablation zones compared to SSM-RFA, thereby providing at least equivalent clinical outcomes compared to SSM-RFA.
The superiority of DSM-RFA over SSM-RFA in inducing larger ablation zones in our study is in good agreement with the results of previous ex vivo and in vivo animal experiments (19,20). However, in our study, the difference in ablation volume did not lead to the difference in LTP rate. Although the two year LTP rate of DSM-RFA (4.3%) was better than that of SSM-RFA (10.1%) (which is similar to the value reported previously by Woo et al. (15) [10%]), this difference in our study was not statistically significant (p = 0.149). Nonetheless, we speculate that there is still a possibility that the DSM-RFA improves LTP rate compared to SSM-RFA in treating HCC. First, as a preliminary study, the number of subjects may not be sufficient to provide appropriate statistical power. Second, the small tumor size (1.54 ± 0.56 cm and 1.66 ± 0.63 cm, respectively, for DSM- and SSM-RFA) would also account for the similar therapeutic results of the two techniques in our study despite of the superior performance demonstrated by DSM-RFA in creating larger ablation volumes. The ratio of Dmi/Dmx in our study (0.76) was slightly smaller than the value reported by Yoon et al. (19) (0.8–0.9). In our study population, 45.4% and 59.6% of the HCCs, treated with the SSM-RFA and DSM-RFA, respectively, were located in the subcapsular portion, where the formation of an ideal ellipsoid ablation zone may be interrupted. Therefore, the minimum diameter of the ablation zone may have been underestimated in our study. Considering the effect of tumor location on the diameter of the ablation zone, and the Dmi of albation zone in our study (3.76 ± 0.85 cm vs. 3.38 ± 0.82 cm, p = 0.002), and safety margins of 5 mm, we suspect that the DSM-RFA may even further improve the clinical outcome of patients with larger tumors, especially 2.5–3.0 cm in size, compared to SSM-RFA (7,8,9). According to recent studies (29,30,31,32,33), combined treatment of RFA and TACE or antiangiogenic treatments seems to provide better results than RFA and TACE alone for the treatment of large HCC (defined as those exceeding 3 cm in size). However, considering the increased complexity and cost of combination treatments compared with RFA, the improved performance of DSM-RFA in creating larger ablation zone demonstrated in our study could hold values for managing patients with 2.5–3.0 cm HCCs. Further studies with a larger study population and tumors of larger sizes are warranted to validate our hypothesis.
In our study, there were only two major complications, one pleural effusion requiring thoracentesis and one pericardial effusion, in the DSM-RFA group, resulting in a major complication rate of 3.8% (2/52). Our study results are similar to the value reported in a previous study on SSM-RFA (4.8%, 8/166) by Woo et al. (15), and the complication rates of 2–3% reported for the single electrode approach (34,35). Moreover, no significant differences were observed in procedure-related complications between the two groups. Previous studies have demonstrated that the multiple electrode approach using various RF energy delivery modes was indeed able to create better therapeutic efficacy than the single electrode approach (13,15,18). However, major complication rates were reported up to 6.9%, which could be related with the increased risk of electrode-related injury such as bleeding or hepatic parenchymal tear (1,13,15,34,36,37,38,39). Based on our study results, the DSM-RFA technique using a separable clustered electrode is safe and similar to other single electrode or multiple electrode approaches (1,13,15,34,36,37,38,39).
Asymptomatic pleural effusion was the most frequently observed side effect in our study. 49.0% (51/104) of the patients who underwent RFA using the artificial ascites technique developed post-procedural asymptomatic pleural effusion, which was similar to the incidence (56%, 14/25) reported by Rhim et al. (25). The possible mechanisms by which pleural effusion develops in patients with ascites include lymphatic drainage system, and micro- or macroscopic diaphragmatic defect (40).
This study has several limitations. First, there is the possibility of selection bias as we only prospectively enrolled patients who had undergone DSM-RFA, whereas patients treated with SSM-RFA were included retrospectively. In order to remove the selection bias, randomized controlled prospective studies are warranted to compare clinical efficacy of DSM-RFA and SSM-RFA. Second, this study included a relatively small number of patients with a short follow-up period of two years. Third, as discussed above, this study included relatively small sized tumors, where the demonstrated advantage of DSM-RFA in inducing a larger ablation volume may have been limited. Therefore, further studies including larger study population and larger sized tumors are warranted to validate the true impact of DSA-RFA on clinical outcomes. Finally, although hepatic failure is a rare complication of RFA and there was no patient who developed hepatic failure in our study, theoretically, the risk of hepatic function deterioration might be higher in DSM-RFA due to its capability to induce larger ablation zones compared to SSM-RFA (15,41). Therefore, DSM-RFA should be performed by experienced ablation specialists with thorough planning of the ablation zone before the procedure, and precise in situ monitoring of the ablation zone during the procedure is mandatory.
In conclusion, the results of our study demonstrated that DSM-RFA using a separable clustered electrode is safe and provides high local tumor control and good preliminary clinical outcome for small HCCs, which are at least comparable to those of SSM-RFA.
Acknowledgments
We thank Myungseok Kim, RT for his technical assistance in the RFA procedures.
Footnotes
This study was supported by research grant from the STARmed Co. (No. 06-2013-2560).
References
- 1.Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015;16:1226–1239. doi: 10.3348/kjr.2015.16.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, et al. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16:301–311. doi: 10.1007/s11605-011-1745-x. discussion 311. [DOI] [PubMed] [Google Scholar]
- 6.Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, et al. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62–67. doi: 10.1016/j.gassur.2004.10.003. discussion 67-68. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 9.Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011;196:W565–W572. doi: 10.2214/AJR.10.5122. [DOI] [PubMed] [Google Scholar]
- 10.Pereira PL. Actual role of radiofrequency ablation of liver metastases. Eur Radiol. 2007;17:2062–2070. doi: 10.1007/s00330-007-0587-0. [DOI] [PubMed] [Google Scholar]
- 11.Ni Y, Mulier S, Miao Y, Michel L, Marchal G. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005;30:381–400. doi: 10.1007/s00261-004-0253-9. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012;13:34–43. doi: 10.3348/kjr.2012.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007;42:163–171. doi: 10.1097/01.rli.0000252495.44818.b3. [DOI] [PubMed] [Google Scholar]
- 15.Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268:589–600. doi: 10.1148/radiol.13121736. [DOI] [PubMed] [Google Scholar]
- 16.Lee ES, Lee JM, Kim WS, Choi SH, Joo I, Kim M, et al. Multiple-electrode radiofrequency ablations using Octopus® electrodes in an in vivo porcine liver model. Br J Radiol. 2012;85:e609–e615. doi: 10.1259/bjr/61619687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol. 2011;22:771–779. doi: 10.1016/j.jvir.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Weisbrod AJ, Atwell TD, Callstrom MR, Farrell MA, Mandrekar JN, Charboneau JW. Percutaneous radiofrequency ablation with a multiple-electrode switching-generator system. J Vasc Interv Radiol. 2007;18:1528–1532. doi: 10.1016/j.jvir.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014;15:235–244. doi: 10.3348/kjr.2014.15.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon JH, Lee JM, Han JK, Choi BI. Dual switching monopolar radiofrequency ablation using a separable clustered electrode: comparison with consecutive and switching monopolar modes in ex vivo bovine livers. Korean J Radiol. 2013;14:403–411. doi: 10.3348/kjr.2013.14.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056–1065. doi: 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- 22.Hope TA, Fowler KJ, Sirlin CB, Costa EA, Yee J, Yeh BM, et al. Hepatobiliary agents and their role in LI-RADS. Abdom Imaging. 2015;40:613–625. doi: 10.1007/s00261-014-0227-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227–239. doi: 10.14366/usg.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min JH, Lim HK, Lim S, Kang TW, Song KD, Choi SY, et al. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol. 2014;20:61–70. doi: 10.3350/cmh.2014.20.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190:91–98. doi: 10.2214/AJR.07.2384. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 29.Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: state of the art. World J Gastroenterol. 2016;22:1935–1942. doi: 10.3748/wjg.v22.i6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyun D, Cho SK, Shin SW, Park KB, Park HS, Choo SW, et al. Early stage hepatocellular carcinomas not feasible for ultrasound-guided radiofrequency ablation: comparison of transarterial chemoembolization alone and combined therapy with transarterial chemoembolization and radiofrequency ablation. Cardiovasc Intervent Radiol. 2016;39:417–425. doi: 10.1007/s00270-015-1194-0. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705.e4. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016;17:93–102. doi: 10.3348/kjr.2016.17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jun HY, Ryu JH, Byun SJ, Jeong CW, Kim TH, Lee YH, et al. Combined radiofrequency ablation and double anti-angiogenic protein therapy to increase coagulation efficacy: an experimental study in a murine renal carcinoma model. Korean J Radiol. 2015;16:776–782. doi: 10.3348/kjr.2015.16.4.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 35.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–418. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 36.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123–134. doi: 10.1148/rg.231025054. discussion 134-136. [DOI] [PubMed] [Google Scholar]
- 37.Seror O, N'Kontchou G, Van Nhieu JT, Rabahi Y, Nahon P, Laurent A, et al. Histopathologic comparison of monopolar versus no-touch multipolar radiofrequency ablation to treat hepatocellular carcinoma within Milan criteria. J Vasc Interv Radiol. 2014;25:599–607. doi: 10.1016/j.jvir.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Seror O, N'Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, et al. Large (>or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes--initial experience in 26 patients. Radiology. 2008;248:288–296. doi: 10.1148/radiol.2481071101. [DOI] [PubMed] [Google Scholar]
- 39.Bruners P, Schmitz-Rode T, Günther RW, Mahnken A. Multipolar hepatic radiofrequency ablation using up to six applicators: preliminary results. Rofo. 2008;180:216–222. doi: 10.1055/s-2008-1027184. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya A, Mittal BR, Biswas T, Dhiman RK, Singh B, Jindal SK, et al. Radioisotope scintigraphy in the diagnosis of hepatic hydrothorax. J Gastroenterol Hepatol. 2001;16:317–321. doi: 10.1046/j.1440-1746.2001.02441.x. [DOI] [PubMed] [Google Scholar]
- 41.Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16346 treated nodules in 13283 patients. Hepatol Res. 2012;42:1058–1064. doi: 10.1111/j.1872-034X.2012.01025.x. [DOI] [PubMed] [Google Scholar]