Abstract
Objective
The study aimed to describe portal stenting for postoperative portal occlusion with delayed (≥ 3 months) variceal bleeding in the afferent jejunal loop.
Materials and Methods
Eleven consecutive patients (age range, 2–79 years; eight men and three women) who underwent portal stenting between April 2009 and December 2015 were included in the study. Preoperative medical history and the postoperative clinical course were reviewed. Characteristics of portal occlusion and details of procedures were also investigated. Technical success, treatment efficacy (defined as disappearance of jejunal varix on follow-up CT), and clinical success were analyzed. Primary stent patency rate was plotted using the Kaplan-Meier method.
Results
All patients underwent hepatobiliary-pancreatic cancer surgery except two children with liver transplantation for biliary atresia. Portal occlusion was caused by benign postoperative change (n = 6) and local tumor recurrence (n = 5). Variceal bleeding occurred at 27 months (4 to 72 months) and portal stenting was performed at 37 months (4 to 121 months), on average, postoperatively. Technical success, treatment efficacy, and clinical success rates were 90.9, 100, and 81.8%, respectively. The primary patency rate of portal stent was 88.9% during the mean follow-up period of 9 months. Neither procedure-related complication nor mortality occurred.
Conclusion
Interventional portal stenting is an effective treatment for delayed jejunal variceal bleeding due to portal occlusion after hepatobiliary-pancreatic surgery.
Keywords: Portal vein obstruction, Jejunal varix, Stent placement
INTRODUCTION
Postoperative variceal bleeding from the afferent jejunal loop is rare and has been reported as a case report or as part of a case series (1,2,3,4,5,6,7). Possible causes include portal venous hypertension in liver cirrhosis and more commonly portal vein (PV) stenosis or occlusion after surgery such as pancreaticoduodenectomy or liver transplantation (LT) (8,9,10,11,12). The reported incidence of postoperative PV occlusion is 19.6% in patients who underwent pancreaticoduodenectomy (8) and between 0.5 and 3% in patients with LT (13,14). Acute PV occlusion or stenosis can be detected early because laboratory tests and radiologic evaluation are frequent in the immediate postoperative period. On the other hand, diagnosis of chronic PV occlusion can be delayed until massive gastrointestinal (GI) bleeding from ectopic varix occurs because of normal liver function and intermittent nature of melena or small amount of hematochezia (2,4,6,15).
Several studies reported that surgical restoration of occluded portal venous blood flow and resection of the bowel segment containing varix are effective (5,16,17). However, surgical approach may be difficult and sometimes impossible because of severe postoperative adhesion and a high risk of bleeding from dilated collateral venous channels. Patients' poor general condition from chronic illness and massive GI bleeding also often preclude surgery. As an alternative to surgery, interventional treatments have been introduced including embolization of ectopic varix with either endovascular route or direct puncture technique (15,18), PV balloon dilatation, or stenting with or without varix embolization (1,2,4,9,10,19). Since the major cause of GI bleeding in this clinical setting is varix caused by PV occlusion, recanalization of occluded PV is fundamental to achieving long-term clinical success (7).
Herein, we reported our experience of PV stenting for postoperative PV occlusion complicating jejunal variceal bleeding in patients who underwent hepatobiliarypancreatic surgery.
MATERIALS AND METHODS
Patients
The Institutional Review Board approved this retrospective study and informed consent was waived. Eleven consecutive patients with delayed (≥ 3 months) GI bleeding from jejunal varix caused by PV occlusion after hepatobiliary-pancreatic surgery who were treated with PV stent placement between April 2009 and December 2015 were included in the study. Of these, eight were male and three were female patients, aged from 2 to 79 years. PV occlusion and jejunal varix were diagnosed based on portal phase images of contrast-enhanced computed tomography (CT). PV occlusion was defined as segmental discontinuation of the PV and development of collateral channels. Jejunal varix was demonstrated by clustered, tortuous, and dilated veins in the wall of the jejunum around the choledochojejunostomy or hepaticojejunostomy. Treatment was determined based on laboratory findings and the clinical situation: decreased serum hemoglobin level (7.7–11.5 g/dL) and GI bleeding that was recurrent and worsening in intensity and duration.
Portal Vein Stenting
The access route for PV stenting was determined based on the anatomic location, shape, and extent of PV occlusion depicted on preprocedural contrast-enhanced CT. The presence of a tapering end in the patent PV or superior mesenteric vein (SMV) or splenic vein (SPV) was a main determinant of the access route. Access was initially performed via right or left transhepatic and/or transsplenic approach. Percutaneous transhepatic and/or transsplenic approach was performed with a 22-G Chiba needle (Cook Incorporated, Bloomington, IN, USA) under ultrasound guidance. After insertion of a 6 Fr or 7 Fr vascular sheath into the intrahepatic PV or SPV, 4 Fr or 5 Fr angiographic catheter (either Torcon NB Advantage catheter [Cook Incorporated] or Glidecath Angled Taper [Terumo Corporation, Tokyo, Japan]) was advanced over a 0.035-inch hydrophilic guidewire. Navigation of the guidewire through the occluded PV was performed under the landmark of surgical clips seen on fluoroscopy or imaginary PV course. When the guidewire passage was difficult, a braided sheath (Super Arrow_Flex, Arrow International, Inc., Reading, PA, USA) was used to achieve better mechanical support. A 2 Fr microcatheter (Progreat, Terumo Corporation) and a microguidewire (0.016 or 0.014 inch in diameter) were also used for difficult cases. After a wire passed through an occluded segment, a catheter was advanced into the SMV or SPV; subsequently, venography was obtained to identify the length of the occluded segment, the diameter of SMV or PV, and any collateral veins including jejunal varix. Along the exchanged stiffer guidewire (Rosen Curved Wire Guide, Cook Incorporated), preballoon dilatation was performed with a 4- to 6-mm diameter balloon and a self-expandable nitinol stent (SMART control, Cordis Corporation, Miami Lakes, FL, USA) was placed to cover the entire occluded segment. Diameter of the stent was 1 to 2 mm larger than the maximum diameter of the remnant PV or SMV demonstrated on angiography. Post-dilatation was performed with an 8- or 10-mm diameter balloon. Pressure gradient was not routinely measured; however, two patients had post-procedural pressure gradient measurement. Completion angiography was performed with the tip of a catheter in the SMV or SPV. One patient underwent aspiration thrombectomy with a 6 Fr guiding catheter (Envoy, Codman & Shurtleff, Inc., Raynham, MA, USA) for a partially protruded thrombus into the stent lumen, which was identified on completion angiogram. Percutaneous access tract in the liver and/or spleen was embolized with coils and/or Gelfoam (Cutanplast, Mascia Brunelli S.P.A, Milano, Italy). Anticoagulation was performed at least 3 months after the procedure with acetylsalicylic acid 100 mg and clopidogrel 75 mg.
Analyses and Follow-Up
Review of electronic medical records was performed for underlying disease, type of surgery, and postoperative treatment. Symptom onset period was between the date of surgery and the date of symptom occurrence. The extent of PV occlusion, presence of tapering end in patent portal or SMV, and the location of ectopic varix were analyzed based on contrast-enhanced CT. The cause of PV occlusion was evaluated with postoperative CT and positron emission tomography. Technical success was defined as complete restoration of the hepatopetal portal venous flow after stent placement on completion angiography. Treatment efficacy was defined as disappearance of jejunal varix on the first follow-up CT after PV stenting. Clinical success was defined as disappearance of GI bleeding without procedure-related complications. Follow-up period was defined as the time-period between the date of interventional treatment and the date of the last hospital visit. Contrast-enhanced CT or Doppler ultrasonography was performed to evaluate stent patency in all patients, except one who was transferred after procedure. Primary patency of the PV stent was calculated with Kaplan-Meier analysis using statistical software (IBM SPSS for Windows, version 20.0, IBM Corp., Armonk, NY, USA). Complication and mortality were evaluated based on the Society of Interventional Radiology guideline (20).
RESULTS
Pretreatment Clinical Course
All patients underwent hepatobiliary-pancreatic surgery for pancreatic cancer except two children with LT for biliary atresia. Surgery included pylorus-preserving pancreaticoduodenectomy (n = 7), total pancreatectomy (n = 1), and Whipple's operation (n = 1). In two patients, SMV resection and anastomosis was performed at the time of surgery. Between surgery and PV stenting, two patients received both radiation treatment and adjuvant chemotherapy; and the other three patients received adjuvant chemotherapy.
Gastrointestinal bleeding occurred, on average, 27 months (4 to 72 months) after surgery. GI bleeding was manifested as melena (n = 6) and hematochezia (n = 5). Initially, hemorrhage was intermittent and small in amount in all patients and progressed in only three patients. Endoscopic examination was performed in nine patients: endoscopic hemostasis was attempted but failed in three patients.
Characteristics of Portal Occlusion and Portal Stenting
The cause of PV occlusion was benign postoperative change in six patients and local tumor recurrence in five patients. All PV occlusion was extrahepatic. In eight out of 11 patients, occlusion extended to the splenoportal junction or SMV. On CT images, the mean length of PV occlusion was 3.7 cm (range, 1.1 to 6.3 cm). Tapering appearance of the patent PV or SPV was seen in eight patients on CT and 10 patients on angiogram (Fig. 1). Varix was clearly depicted on CT in the afferent jejunal loop in all patients. Percutaneous transhepatic PV stenting was performed, on average, 37 months (4 to 121 months) after surgery. Jejunal varix embolization was not performed in all patients. Transhepatic and transsplenic approaches were used in 10 patients (right in eight patients and left in two patients) and in one patient, respectively. The approach was determined from the presence of tapering appearance in patent vein on CT (Supplementary Movie 1–4 in the online-only Data Supplement); otherwise, transhepatic approach was attempted first. Post-stent pressure gradient was measured in two patients and was less than 3 mm Hg between SMV and the intrahepatic PV.
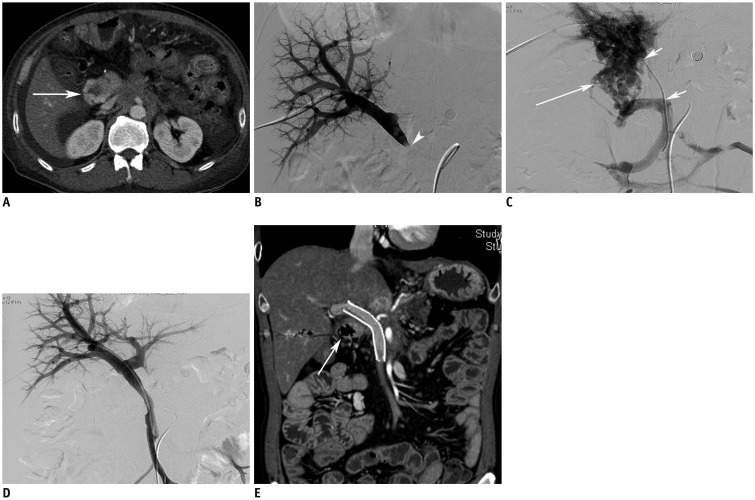
Fig. 1. 53-year-old man with hematochezia.
Patient underwent pylorus-preserving pancreaticoduodenectomy due to pancreatic cancer 737 days ago.
A. Axial image shows varix in afferent jejunal loop (arrow). B, C. Direct portogram (B) via transhepatic approach shows occlusion of main portal vein (arrowhead). Superior mesenteric venogram (C) demonstrates extensive collateral channels along afferent jejunal loop (arrow) and segmental occlusion of portal vein (smaller arrows). D. Direct venogram after deployment of stent (12 mm in diameter and 80 mm in length) shows disappearance of collateral channels and opacification of both portal veins. E. Follow-up CT performed 60 days after portal stenting shows patent stent and disappearance of varix in afferent jejunal loop (arrow).
Outcomes of Portal Stenting and Complication
Technical success rate was 90.9% (10 out of 11). PV stenting was successful at first trial in nine patients and at second trial in two patients. PV stenting failed in one patient who had undergone radiation therapy for local cancer recurrence. Mean follow-up period was 9 months (range, 1–27 months). Follow-up CT in seven patients and Doppler ultrasonography in three patients were performed at mean 7 months (range, 1–14 months) and 12 months (range, 4–26 months) after PV stenting, respectively. Treatment efficacy was 100%. Jejunal varix disappeared on post-stenting CT available in six patients with successful PV stenting and remained in one patient with technical failure. Clinical success rate was 81.8% (9 out of 11). One patient visited the emergency room 40 days after PV stenting because of hematochezia. This patient had a long segmental occlusion from the PV to SMV and a history of radiation therapy before procedure. Although portal venous flow was restored after placement of two nitinol stents, completion angiogram showed partial thrombi within the PV. Despite the successful aspiration thrombectomy, good portal venous flow on final angiogram, and the dual antiplatelet medication after procedure, the patient visited the emergency room due to recurred hematochezia. His follow-up CT revealed complete obstruction of the PV stent and recurred jejunal varix. Primary patency rate of the PV stent during the mean follow-up period of 9 months was 88.9% (Fig. 2). Neither procedure-related complication nor mortality occurred during the follow-up period. Cases are summarized in Table 1.
Fig. 2. Primary patency rate of portal stent.
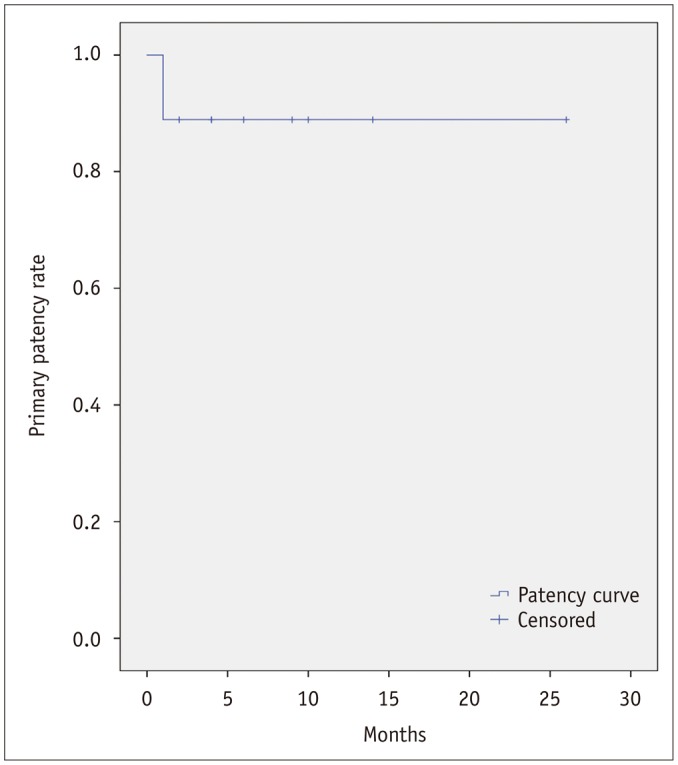
Table 1. Summary of Eleven Cases.
| No. | Sex/Age | Op Name | Cause of Portal Occlusion | Time to Stenosis (Month) | Time to Occlusion (Month) | Time to Bleeding (Month) | Time to Portal Stenting (Month) | Technical Success | Technique Efficacy | Clinical Success | Follow-Up Period (Month) | Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/53 | PPPD | Recurrence | 20 | 26 | 24 | 30 | Yes | Yes | Yes | 15 | No |
| 2 | M/54 | PPPD | Benign | 1 | 2 | 7 | 10 | Yes | NA | Yes | 27 | No |
| 3 | M/50 | TP* | Benign | 24 | 25 | 24 | 25 | Yes | Yes | Yes | 10 | No |
| 4 | M/52 | PPPD | Benign | 3 | 8 | 7 | 56 | Yes | Yes | Yes | 12 | No |
| 5 | M/68 | PPPD* | Recurrence | 1 | 2 | 4 | 4 | No | NA | NA | 7 | No |
| 6 | M/78 | PPPD | Benign | 1 | 5 | 5 | 5 | Yes | Yes | Yes | 5 | No |
| 7 | M/79 | Whipple | Recurrence | 1 | 3 | 55 | 55 | Yes | Yes | Failure | 2 | No |
| 8 | M/77 | PPPD | Recurrence | 55 | NA | 64 | 65 | Yes | Yes | Yes | 4 | No |
| 9 | F/68 | PPPD | Recurrence | 3 | 11 | 19 | 22 | Yes | NA | Yes | 1 | No |
| 10 | F/10 | LT | Benign | 10 | 22 | 72 | 121 | Yes | NA | Yes | 9 | No |
| 11 | F/2 | LT | Benign | NA | 16 | 18 | 20 | Yes | NA | Yes | 3 | No |
*Superior mesenteric vein resection and anastomosis was performed as well. F = female, LT = liver transplantation, M = male, Op = operation, PPPD = pylorus preserving pancreaticoduodenectomy, TP = total pancreatectomy
DISCUSSION
Ectopic varices refer to any abnormal large venous collateral in the abdomen except in the cardioesophageal area from portal hypertension (21). In the postoperative period, possible causes are extrahepatic PV occlusion or stenosis from cancer recurrence or benign postoperative change (2,3,4,6,10). In PV stenosis or occlusion, splanchnic venous blood drains to the systemic veins or intrahepatic PVs through collateral veins forming an ectopic varix in the duodenum, jejunum, or stomas (21). Among them, jejunal ectopic varix seems to develop frequently after hepatobiliary-pancreatic surgery. Collateral channels are likely to develop via low-resistant natural vascular space such as pancreaticoduodenal vein or gastrocolic vein along the afferent jejunal loop rather than newly formed postoperative tissue around the hepatic hilum. Patients can be asymptomatic initially. However, as the PV pressure rises, intermittent hematochezia or melena progresses to copious GI bleeding originating from the jejunal varix. Regarding diagnosis, endoscopy plays a limited role because it cannot reach up to the afferent jejunal loop in the postoperative state. On the other hand, contrast-enhanced CT depicts both PV status (stenosis or occlusion) and ectopic jejunal varix.
Treatment can focus on either jejunal varix itself or portal decompression depending on the clinical situation. Surgical removal of jejunal varix was attempted (22,23). Endovascular treatment includes sclerosis of jejunal varix (1,5,7). However, varix treatment leaves portal hypertension unresolved so that any ectopic intestinal varix may recur (21). Therefore, the fundamental point of treatment is PV decompression with either surgical shunt operation or endovascular PV recanalization. Surgical shunts between the SMV (or the SPV) and intrahepatic left PV were attempted to relieve portal hypertension but were invasive (5,24,25). On the other hand, the endovascular approach is simple and less invasive. In general, transjugular intrahepatic portosystemic shunt or balloon-occluded retrograde transvenous obliteration is not indicated in complete portal occlusion (26). Some authors reported successful interventional portal recanalization in patients with postoperative PV occlusion instead (2,3,4,6). However, our study demonstrated not only successful recanalization (90.9% technical success) of chronically occluded extrahepatic PVs but also resolution of ectopic jejunal varix without embolization, suggesting that PV stenting is effective in PV decompression and varix resolution even in patients with bleeding.
Success of PV recanalization seems to be associated with presence of tapering appearance of the patent vein. All successful passages of a guidewire were possible through the tapering end of the patent vein in our study. Careful analysis of preprocedural contrast-enhanced CT and angiogram during procedure may play a vital role in determining the approach direction and success of recanalization. Other possible factors may be postoperative radiation treatment, length of occluded segment, malignant PV invasion, and presence of thrombotic component. One patient failed to pass a guidewire through the long segmental occlusion extending from PV to SMV after radiation therapy due to cancer recurrence. The other one patient with clinical failure had a long segmental occlusion from cancer recurrence; although the stent was placed successfully, stent occlusion and rebleeding occurred eventually. Radiation therapy seems to make the soft tissue around the occluded PV harder so that wire passage becomes extremely difficult. Study on a larger sample size is required to confirm the possible prognostic factors.
Procedure-related complications are rare (4,9). Reported complications include hemothorax from the hepatic puncture site, pleural effusion, and unsatisfactory positioning of a stent. No procedure-related mortality has been reported so far.
Our study has several limitations. First, the current study is a small retrospective study. Therefore, the patient group is heterogeneous in terms of patient age, type of operation, underlying disease, cause of portal obstruction, and history of radiation treatment. Thus, technical success, technique efficacy, clinical success, and patency rate can be biased. Second, longer a follow-up period is required to evaluate the long-term physiologic effect and determine the patency of PV stenting. Not all the patients underwent pressure gradient measurement before and after portal stenting. PV decompression was considered based on the disappearance of ectopic jejunal varix on follow-up CT.
In conclusion, interventional PV stenting can be attempted as a treatment for delayed (≥ 3 months) jejunal variceal bleeding with portal occlusion after hepatobiliarypancreatic surgery.
Supplementary Movie Legends
Preoperative axial CT images show segmental occlusion of the main portal vein.
Preoperative coronal CT images show that tapering appearance of the main portal vein is traced from the patent splenic vein.
Direct portogram via transhepatic approach shows occlusion of the main portal vein with blind pouch.
Venogram via transsplenic approach shows tapering end of the main portal vein.
References
- 1.Choi JW, Kim HC, Jae HJ, Jung HS, Hur S, Lee M, et al. Transcatheter embolotherapy with N-butyl cyanoacrylate for ectopic varices. Cardiovasc Intervent Radiol. 2015;38:344–351. doi: 10.1007/s00270-014-0943-9. [DOI] [PubMed] [Google Scholar]
- 2.Hiraoka K, Kondo S, Ambo Y, Hirano S, Omi M, Okushiba S, et al. Portal venous dilatation and stenting for bleeding jejunal varices: report of two cases. Surg Today. 2001;31:1008–1011. doi: 10.1007/s005950170013. [DOI] [PubMed] [Google Scholar]
- 3.Mathias K, Bolder U, Löhlein D, Jäger H. Percutaneous transhepatic angioplasty and stent implantation for prehepatic portal vein obstruction. Cardiovasc Intervent Radiol. 1993;16:313–315. doi: 10.1007/BF02629164. [DOI] [PubMed] [Google Scholar]
- 4.Ota S, Suzuki S, Mitsuoka H, Unno N, Inagawa S, Takehara Y, et al. Effect of a portal venous stent for gastrointestinal hemorrhage from jejunal varices caused by portal hypertension after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2005;12:88–92. doi: 10.1007/s00534-004-0941-4. [DOI] [PubMed] [Google Scholar]
- 5.Saeki Y, Ide K, Kakizawa H, Ishikawa M, Tashiro H, Ohdan H. Controlling the bleeding of jejunal varices formed at the site of choledochojejunostomy: report of 2 cases and a review of the literature. Surg Today. 2013;43:550–555. doi: 10.1007/s00595-012-0243-4. [DOI] [PubMed] [Google Scholar]
- 6.Sakai M, Nakao A, Kaneko T, Takeda S, Inoue S, Yagi Y, et al. Transhepatic portal venous angioplasty with stenting for bleeding jejunal varices. Hepatogastroenterology. 2005;52:749–752. [PubMed] [Google Scholar]
- 7.Sato T, Yasui O, Kurokawa T, Hashimoto M, Asanuma Y, Koyama K. Jejunal varix with extrahepatic portal obstruction treated by embolization using interventional radiology: report of a case. Surg Today. 2003;33:131–134. doi: 10.1007/s005950300029. [DOI] [PubMed] [Google Scholar]
- 8.Kang MJ, Jang JY, Chang YR, Jung W, Kim SW. Portal vein patency after pancreatoduodenectomy for periampullary cancer. Br J Surg. 2015;102:77–84. doi: 10.1002/bjs.9682. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Yang W, Huang Q, Gao K, Wei B, Zhai R, et al. Interventional treatment for portal venous occlusion after liver transplantation: long-term follow-up results. Medicine (Baltimore) 2015;94:e356. doi: 10.1097/MD.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang S, Sung KB, Park YH, Jung DH, Lee SG. Portal vein stenting for portal hypertension caused by local recurrence after pancreatoduodenectomy for periampullary cancer. J Gastrointest Surg. 2007;11:333–337. doi: 10.1007/s11605-006-0058-y. [DOI] [PubMed] [Google Scholar]
- 11.Ko GY, Sung KB, Lee S, Yoon HK, Kim KR, Kim KM, et al. Stent placement for the treatment of portal vein stenosis or occlusion in pediatric liver transplant recipients. J Vasc Interv Radiol. 2007;18:1215–1221. doi: 10.1016/j.jvir.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Park KB, Choo SW, Do YS, Shin SW, Cho SG, Choo IW. Percutaneous angioplasty of portal vein stenosis that complicates liver transplantation: the mid-term therapeutic results. Korean J Radiol. 2005;6:161–166. doi: 10.3348/kjr.2005.6.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannuri U, Velhote MC, Santos MM, Gibelli NE, Ayoub AA, Maksoud-Filho JG, et al. Pediatric liver transplantation: fourteen years of experience at the children institute in SãoPaulo, Brazil. Transplant Proc. 2004;36:941–942. doi: 10.1016/j.transproceed.2004.03.101. [DOI] [PubMed] [Google Scholar]
- 14.Carnevale FC, de Tarso Machado A, Moreira AM, Dos Santos AC, da Motta-Leal-Filho JM, Suzuki L, et al. Long-term results of the percutaneous transhepatic venoplasty of portal vein stenoses after pediatric liver transplantation. Pediatr Transplant. 2011;15:476–481. doi: 10.1111/j.1399-3046.2011.01481.x. [DOI] [PubMed] [Google Scholar]
- 15.Saad WE, Saad NE, Koizumi J. Stomal varices: management with decompression tips and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:176–184. doi: 10.1053/j.tvir.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Eriguchi N, Aoyagi S, Hara M, Miyazaki T, Tanaka M, Toyonaga A. Jejunal varices as a cause of massive gastrointestinal bleeding--a case report. Kurume Med J. 1998;45:227–230. doi: 10.2739/kurumemedj.45.227. [DOI] [PubMed] [Google Scholar]
- 17.Yuki N, Kubo M, Noro Y, Kasahara A, Hayashi N, Fusamoto H, et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517. [PubMed] [Google Scholar]
- 18.Sasamoto A, Kamiya J, Nimura Y, Nagino M. Successful embolization therapy for bleeding from jejunal varices after choledochojejunostomy: report of a case. Surg Today. 2010;40:788–791. doi: 10.1007/s00595-009-4129-z. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai K, Amano R, Yamamoto A, Nishida N, Matsutani S, Hirata K, et al. Portal vein stenting to treat portal vein stenosis in a patient with malignant tumor and gastrointestinal bleeding. Int Surg. 2014;99:91–95. doi: 10.9738/INTSURG-D-13-00128.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 21.Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology. 1998;28:1154–1158. doi: 10.1002/hep.510280434. [DOI] [PubMed] [Google Scholar]
- 22.Moncure AC, Waltman AC, Vandersalm TJ, Linton RR, Levine FH, Abbott WM. Gastrointestinal hemorrhage from adhesion-related mesenteric varices. Ann Surg. 1976;183:24–29. doi: 10.1097/00000658-197601000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lein BC, McCombs PR. Bleeding varices of the small bowel as a complication of pancreatitis: case report and review of the literature. World J Surg. 1992;16:1147–1149. doi: 10.1007/BF02067083. discussion 1150. [DOI] [PubMed] [Google Scholar]
- 24.Bambini DA, Superina R, Almond PS, Whitington PF, Alonso E. Experience with the Rex shunt (mesenterico-left portal bypass) in children with extrahepatic portal hypertension. J Pediatr Surg. 2000;35:13–18. doi: 10.1016/s0022-3468(00)80005-6. discussion 18-19. [DOI] [PubMed] [Google Scholar]
- 25.Chen VT, Wei J, Liu YC. A new procedure for management of extrahepatic portal obstruction. Proximal splenic-left intrahepatic portal shunt. Arch Surg. 1992;127:1358–1360. doi: 10.1001/archsurg.1992.01420110106021. [DOI] [PubMed] [Google Scholar]
- 26.Saad WE, Kitanosono T, Koizumi J, Hirota S. The conventional balloon-occluded retrograde transvenous obliteration procedure: indications, contraindications, and technical applications. Tech Vasc Interv Radiol. 2013;16:101–151. doi: 10.1053/j.tvir.2013.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative axial CT images show segmental occlusion of the main portal vein.
Preoperative coronal CT images show that tapering appearance of the main portal vein is traced from the patent splenic vein.
Direct portogram via transhepatic approach shows occlusion of the main portal vein with blind pouch.
Venogram via transsplenic approach shows tapering end of the main portal vein.