Abstract
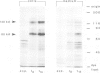
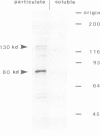
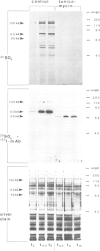
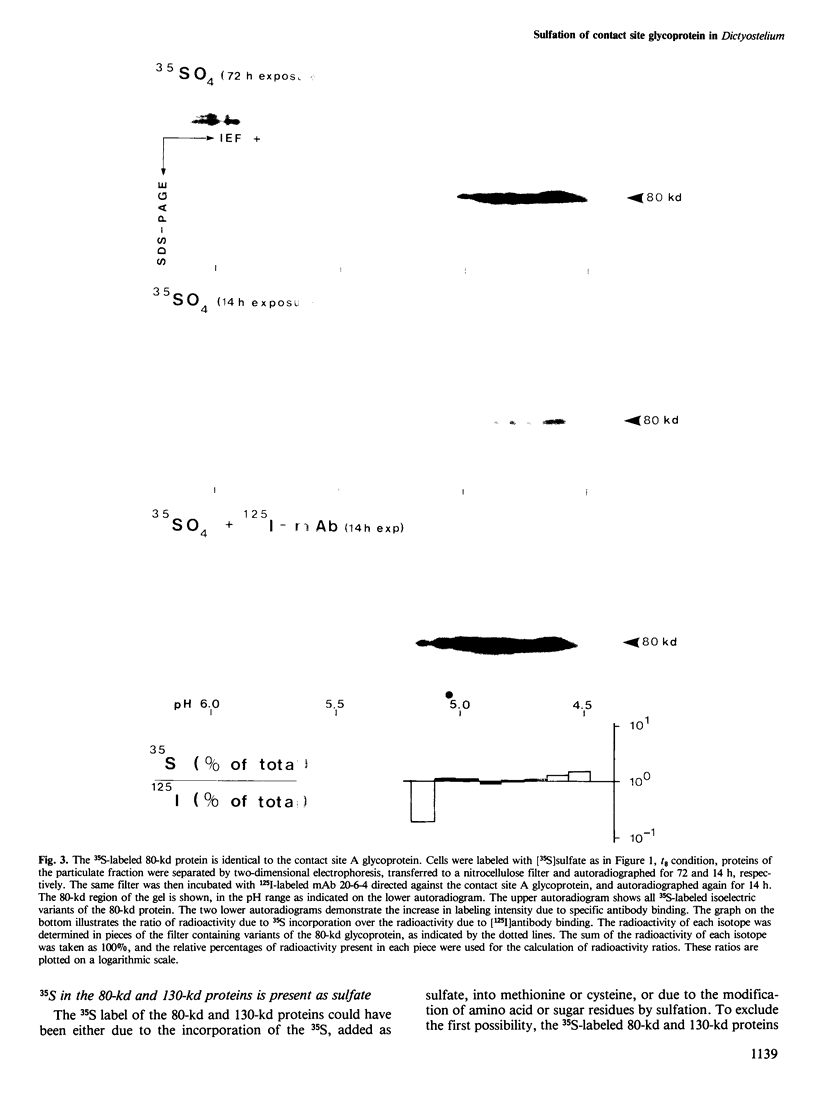
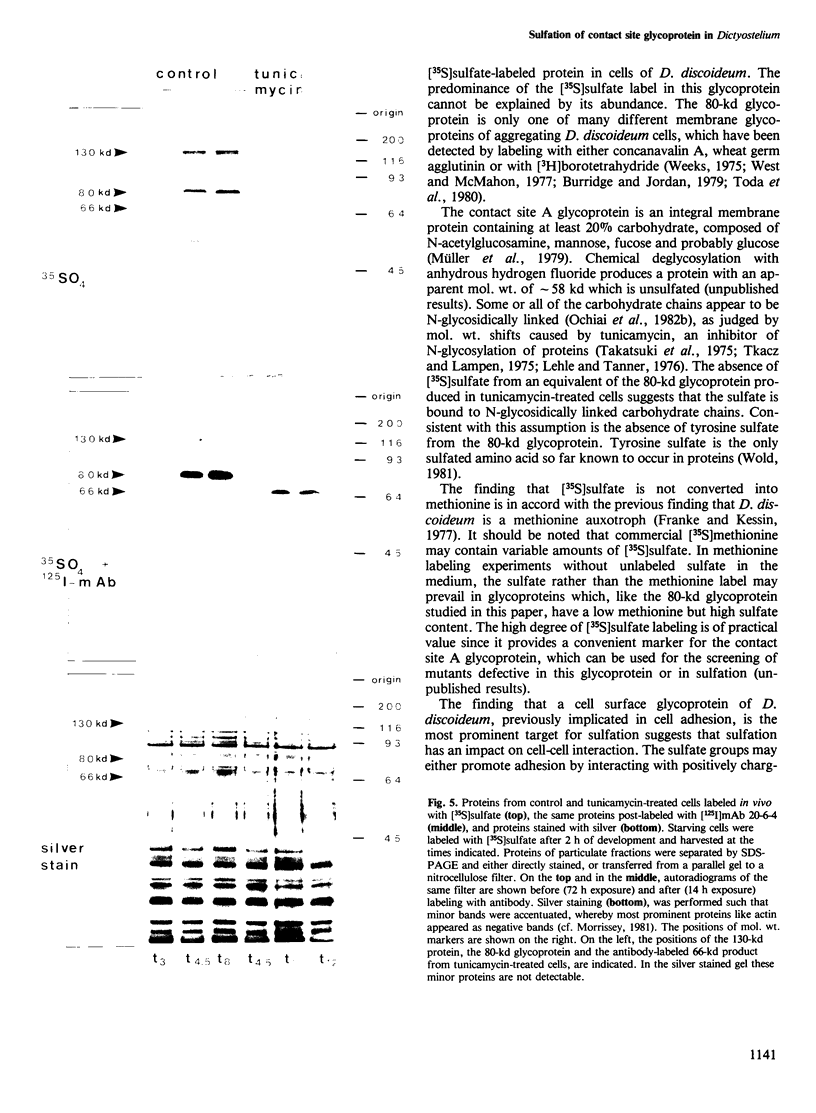
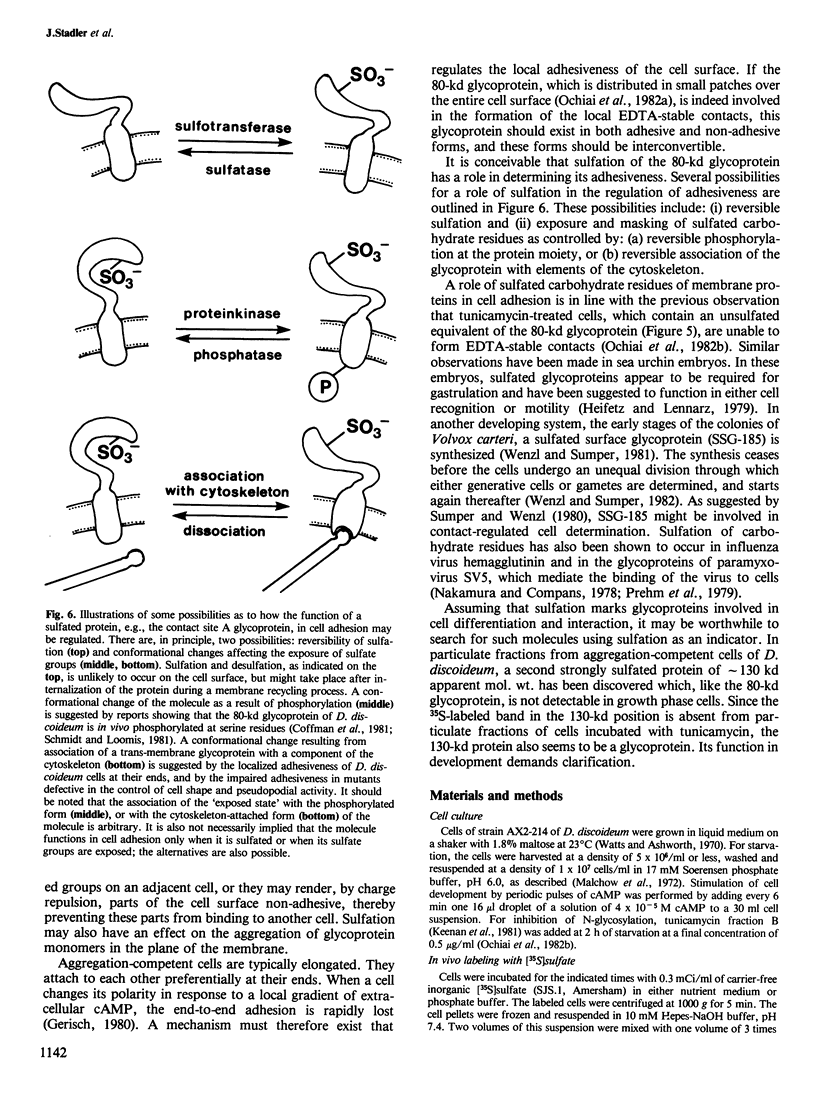
During the development of Dictyostelium discoideum from the growth phase to the aggregation stage, a glycoprotein with an apparent mol. wt. of 80 kd is known to be expressed on the cell surface. This glycoprotein, referred to as contact site A, has been implicated in the formation of species-specific, EDTA-stable contacts of aggregating cells. When developing cells were labeled in vivo with [35S]sulfate, the 80-kd glycoprotein was found to be the most prominently sulfated protein. Another strongly sulfated protein had an apparent mol. wt. of 130 kd and was, like the 80-kd glycoprotein, developmentally regulated and associated with the particulate fraction of the cells. The [35S]sulfate incorporated into the 80-kd and 130-kd proteins was not present as tyrosine-O-sulfate, a modified amino acid found in many proteins of mammalian cells. D. discoideum cells incubated with [35S]sulfate in the presence of tunicamycin, an inhibitor of N-glycosylation, produced a 66-kd protein that reacted with monoclonal antibodies raised against the 80-kd glycoprotein, but no longer contained [35S]sulfate. These results suggest that sulfation of the 80-kd glycoprotein occurred on carbohydrate residues. The possible importance of sulfation for a role of the 80-kd glycoprotein in cell adhesion is discussed.
Keywords: glycoprotein, sulfation, cell adhesion, Dictyostelium
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Gerisch G., Kempff S., Riedel V., Cremer G. Specific inhibition of cell contact formation in Dictyostelium by univalent antibodies. Exp Cell Res. 1970 Nov;63(1):147–158. doi: 10.1016/0014-4827(70)90343-5. [DOI] [PubMed] [Google Scholar]
- Beug H., Katz F. E., Gerisch G. Dynamics of antigenic membrane sites relating to cell aggregation in Dictyostelium discoideum. J Cell Biol. 1973 Mar;56(3):647–658. doi: 10.1083/jcb.56.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Jordan L. The glycoproteins of Dictyostelium discoideum. Changes during development. Exp Cell Res. 1979 Nov;124(1):31–38. doi: 10.1016/0014-4827(79)90254-4. [DOI] [PubMed] [Google Scholar]
- Coffman D. S., Leichtling B. H., Rickenberg H. V. Phosphoproteins in Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;15(4):369–385. doi: 10.1002/jsscb.1981.380150407. [DOI] [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Fromm H., Huesgen A., Wick U. Control of cell-contact sites by cyclic AMP pulses in differentiating Dictyostelium cells. Nature. 1975 Jun 12;255(5509):547–549. doi: 10.1038/255547a0. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Lennarz W. J. Effects of tunicamycin on the biosynthesis of glycosaminoglycans by embryonic chick cornea. J Biol Chem. 1978 Aug 25;253(16):5795–5801. [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Keenan R. W., Hamill R. L., Occolowitz J. L., Elbein A. D. Biological activities of isolated tunicamycin and streptovirudin fractions. Biochemistry. 1981 May 12;20(10):2968–2973. doi: 10.1021/bi00513a039. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Malchow D., Nägele B., Schwarz H., Gerisch G. Membrane-bound cyclic AMP phosphodiesterase in chemotactically responding cells of Dictyostelium discoideum. Eur J Biochem. 1972 Jun 23;28(1):136–142. doi: 10.1111/j.1432-1033.1972.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Yee L. D., Loomis W. F. Immunological analysis of glycoprotein (contact sites A) involved in intercellular adhesion of Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;17(3):197–211. doi: 10.1002/jsscb.380170302. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G., Fromme I., Mayer H., Tsugita A. A membrane glycoprotein of aggregating Dictyostelium cells with the properties of contact sites. Eur J Biochem. 1979 Sep;99(2):419–426. doi: 10.1111/j.1432-1033.1979.tb13271.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Glycopeptide components of influenza viral glycoproteins. Virology. 1978 May 15;86(2):432–442. doi: 10.1016/0042-6822(78)90083-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Schwarz H., Merkl R., Wagle G., Gerisch G. Stage-specific antigens reacting with monoclonal antibodies against contact site A, a cell-surface glycoprotein of Dictyostelium discoideum. Cell Differ. 1982 Jan;11(1):1–13. doi: 10.1016/0045-6039(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Stadler J., Westphal M., Wagle G., Merkl R., Gerisch G. Monoclonal antibodies against contact sites A of Dictyostelium discoideum: detection of modifications of the glycoprotein in tunicamycin-treated cells. EMBO J. 1982;1(8):1011–1016. doi: 10.1002/j.1460-2075.1982.tb01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish R. W., Schmidlin S., Parish C. R. Detection of developmentally controlled plasma membrane antigens of Dictyostelium discoideum cells in SDS-polyacrylamide gels. FEBS Lett. 1978 Nov 15;95(2):366–370. doi: 10.1016/0014-5793(78)81031-x. [DOI] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Schmidt J. A., Loomis W. F. Phosphorylation of the contact site A glycoprotein (gp80) of Dictyostelium discoideum. Dev Biol. 1982 Jun;91(2):296–304. doi: 10.1016/0012-1606(82)90036-7. [DOI] [PubMed] [Google Scholar]
- Stadler J., Bordier C., Lottspeich F., Henschen A., Gerisch G. Improved purification and N-terminal amino acid sequence determination of the contact site A glycoprotein of Dictyostelium discoideum. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):771–776. doi: 10.1515/bchm2.1982.363.2.771. [DOI] [PubMed] [Google Scholar]
- Sumper M., Wenzl S. Sulphation-desulphation of a membrane component proposed to be involved in control of differentiation in Volvox carteri. FEBS Lett. 1980 Jun 2;114(2):307–312. doi: 10.1016/0014-5793(80)81140-9. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Toda K., Ono K., Ochiai H. Surface labeling of membrane glycoproteins and their drastic changes during development of Dictyostelium discoideum. Eur J Biochem. 1980 Oct;111(2):377–388. doi: 10.1111/j.1432-1033.1980.tb04951.x. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks G. Studies of the cell surface of Dictyostelium discoideum during differentiation. The binding of 125I-concanavalin A to the cell surface. J Biol Chem. 1975 Sep 10;250(17):6706–6710. [PubMed] [Google Scholar]
- Wenzl S., Sumper M. Sulfation of a cell surface glycoprotein correlates with the developmental program during embryogenesis of Volvox carteri. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3716–3720. doi: 10.1073/pnas.78.6.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., McMahon D. Identification of concanavalin A receptors and galactose-binding proteins in purified plasma membranes of Dictyostelium discoideum. J Cell Biol. 1977 Jul;74(1):264–273. doi: 10.1083/jcb.74.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]