Abstract
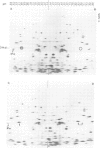
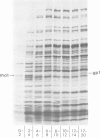
The T4 mot gene regulates middle mode RNA synthesis in phage-infected cells. The mot gene product has been identified in two ways. (i) Infections with amber and temperature-sensitive mot mutants both lead to the disappearance of a number of protein bands on SDS-polyacrylamide gels. These are middle mode proteins whose synthesis depends on mot function. The mot protein disappears from such gels after infection with a mot amber mutant, but not with the mot missense mutant. (ii) This same protein is the only one to have a charge alteration when proteins from wild-type phage and mot missense mutant infections are compared by two-dimensional gel electrophoresis. Mot protein is basic and has a mol. wt. of 24 000. It migrates between the positions of gp 1 and gp IPIII on 15% SDS-polyacrylamide gels. Mot protein synthesis begins immediately after infection and continues until 4 min after infection at 30 degrees C, after which time it is strongly inhibited. This inhibition depends neither on T4 DNA synthesis nor on ADP ribosylation of the alpha subunits of the Escherichia coli RNA polymerase. The mot protein does not regulate its own biosynthesis. It is stable throughout the course of infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black L. W. Bacteriophage T4 internal protein mutants: isolation and properties. Virology. 1974 Jul;60(1):166–179. doi: 10.1016/0042-6822(74)90374-2. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Cardillo T. S., Landry E. F., Wiberg J. S. regA protein of bacteriophage T4D: identification, schedule of synthesis, and autogenous regulation. J Virol. 1979 Dec;32(3):905–916. doi: 10.1128/jvi.32.3.905-916.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C. J., Hsiao C. L., Coon P., Black L. W. Identification and perperties of bacteriophage T4 capsid-formation gene products. J Mol Biol. 1977 Mar 5;110(3):585–601. doi: 10.1016/s0022-2836(77)80113-7. [DOI] [PubMed] [Google Scholar]
- Chace K. V., Hall D. H. Characterization of new regulatory mutants of bacteriophage T4. II. New class of mutants. J Virol. 1975 Apr;15(4):929–945. doi: 10.1128/jvi.15.4.929-945.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Seasholtz A. F. Identification of some bacteriophage T4 prereplicative proteins on two-dimensional gel proteins. J Virol. 1982 Jun;42(3):767–772. doi: 10.1128/jvi.42.3.767-772.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daegelen P., D'Aubenton-Carafa Y., Brody E. The role of rho in bacteriophage T4 development. II. mot-dependent (middle mode) RNA synthesis. Virology. 1982 Feb;117(1):121–134. doi: 10.1016/0042-6822(82)90512-8. [DOI] [PubMed] [Google Scholar]
- Goff C. G., Setzer J. ADP ribosylation of Escherichia coli RNA polymerase is nonessential for bacteriophage T4 development. J Virol. 1980 Jan;33(1):547–549. doi: 10.1128/jvi.33.1.547-549.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb A. Changes in the promoter range of RNA polymerase resulting from bacteriophage T4-induced modification of core enzyme. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3454–3458. doi: 10.1073/pnas.78.6.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb A., Palm P. Control of promoter utilization by bacteriophage T4-induced modification of RNA polymerase alpha subunit. Nucleic Acids Res. 1981 Oct 10;9(19):4863–4878. doi: 10.1093/nar/9.19.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Snyder R. D. Suppressors of mutations in the rII gene of bacteriophage T4 affect promoter utilization. Genetics. 1981 Jan;97(1):1–9. doi: 10.1093/genetics/97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Sauerbier W. Two modes of in vivo transcription for genes 43 and 45 of phage T4. J Virol. 1974 Aug;14(2):341–348. doi: 10.1128/jvi.14.2.341-348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- Karam J., Gold L., Singer B. S., Dawson M. Translational regulation: identification of the site on bacteriophage T4 rIIB mRNA recognized by the regA gene function. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4669–4673. doi: 10.1073/pnas.78.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson T., Richardson J., Goodin D. Mutant of bacteriophage T4D affecting expression of many early genes. Nature. 1974 Jul 5;250(461):48–50. doi: 10.1038/250048a0. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Epstein R. H. Isolation and characterization of conditional lethal mutations in the mot gene of bacteriophage T4. J Mol Biol. 1978 Dec 15;126(3):551–570. doi: 10.1016/0022-2836(78)90058-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pulitzer J. F., Coppo A., Caruso M. Host--virus interactions in the control of T4 prereplicative transcription. II. Interaction between tabC (rho) mutants and T4 mot mutants. J Mol Biol. 1979 Dec 25;135(4):979–997. doi: 10.1016/0022-2836(79)90523-0. [DOI] [PubMed] [Google Scholar]
- Ratner D. Letter to the editor: Bacteriophage T4 transcriptional control gene 55 codes for a protein bound to Escherichia coli RNA polymerase. J Mol Biol. 1974 Nov 15;89(4):803–807. doi: 10.1016/0022-2836(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Schmidt D. A., Mazaitis A. J., Kasai T., Bautz E. K. Involvement of a phage T4 sigma factor and an anti-terminator protein in the transcription of early T4 genes in vivo. Nature. 1970 Mar 14;225(5237):1012–1016. doi: 10.1038/2251012a0. [DOI] [PubMed] [Google Scholar]
- Stevens A. Deoxyribonucleic acid dependent ribonucleic acid polymerases from two T4 phage-infected systems. Biochemistry. 1974 Jan 29;13(3):493–503. doi: 10.1021/bi00700a015. [DOI] [PubMed] [Google Scholar]
- Thermes C., Daegelen P., De Franciscis V., Brody E. In vitro system for induction of delayed early RNA of bacteriophage T4. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2569–2573. doi: 10.1073/pnas.73.8.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Franciscis V., Brody E. In vitro system for middle T4 RNA. I. Studies with Escherichia coli RNA polymerase. J Biol Chem. 1982 Apr 25;257(8):4087–4096. [PubMed] [Google Scholar]
- de Franciscis V., Favre R., Uzan M., Leautey J., Brody E. In vitro system for middle T4 RNA. II. Studies with T4-modified RNA polymerase. J Biol Chem. 1982 Apr 25;257(8):4097–4101. [PubMed] [Google Scholar]