Abstract
To obtain an in-depth understanding of brain diseases, including neurodegenerative diseases, psychiatric illnesses, and neoplasms, scientific approach and verification using postmortem human brain tissue with or without disease are essential. Compared to other countries that have run brain banks for decades, South Korea has limited experience with brain banking; nationwide brain banks started only recently. The goal of this study is to provide provisional guidelines for brain autopsy for hospitals and institutes that have not accumulated sufficient expertise. We hope that these provisional guidelines will serve as a useful reference for pathologists and clinicians who are involved and interested in the brain bank system. Also, we anticipate updating the provisional guidelines in the future based on collected data and further experience with the practice of brain autopsy in South Korea.
Keywords: Dementia, neuropathology, guideline, standardization
Postmortem human brain tissues are critical for the advancement of neuroscience. In basic science dealing with neurochemistry and signaling pathways as well as translational research pursuing therapeutic goals, the final step is often ver-ification through human brain tissues. Obtainment and storage of human brain tissues with reliable medical records and clinical data are invaluable for neuroscience. Antemortem workup using relevant biomarkers and imaging techniques can result in clinical diagnosis, even to the level of specific disorder subtypes. Indeed, brain autopsy remains the gold standard for confirmative diagnosis of neurodegenerative disorders.1 Adequate categorization of postmortem brain tissue according to the final pathologic diagnosis expedites proper use of limited human materials.
In 2016, we established the national neuropathology reference and diagnostic laboratory for Alzheimer's disease (AD). This project aimed to standardize a neuropathology-based diagnosis of dementia by establishing a country-wide brain tissue bank network. Herein, we reviewed brain autopsy procedures performed in brain banks within and outside the co-untry, and also designed an optimal brain autopsy procedure to be performed for brain bank in South Korea. This proposed guideline covers the overall processes of brain autopsy, diagnostic processes, and quality control of stored tissues. The ethical, legal, and procedural issues associated with disbursement of postmortem human brain tissue are not considered, because these issues are beyond the scope of this article. We rather focused on the methodology for management and pa-thologic workup of autopsied human brains, and the section below provides recommendations about the acquisition of human brain tissue, dissection and processing of the tissue, his-tological evaluation to reach a pathologic diagnosis, and quality control of the tissue.
Dissection and preparation of frozen tissue
A brain donated to the brain bank should be processed as soon as possible after death in order to minimize autolytic effects during the postmortem interval. Degenerative processes in the brain are believed to begin at death, but a large body of evidence indicates that the effects of autolysis within 24 hours following death are smaller than antemortem hypoxic events.2,3,4 After removal of the brain, it should be examined, photographed, and weighed. Surface pH should be measured with a pH meter if available. Next, the brain should be separated into two hemispheres. In general, the dominant hemisphere (the left hemisphere for a right-handed person) should be fixed for thorough neuropathologic evaluation, while the contralateral hemisphere should be left unfixed, dissected into slabs, and immediately snap-frozen. However, if a unilateral lesion or more severe lesion with asymmetric changes is identified on gross examination, the side involved should be reserved for pathologic workup and fixed in formalin.2
Before the two hemispheres are separated, the brainstem and cerebellum need to be removed from the cerebrum. To se-parate the brainstem, the mammillary body should be pressed softly with a cutting blade, and the midbrain is detached from the cerebral hemisphere transversely through the crus cerebri up to the dorsal aspect of the superior colliculus.2 When the red nuclei and substantia nigra are clearly visible, the cutting level is appropriate. The cerebellum should be separated from the brainstem by sequential sectioning through the superior, middle, and inferior cerebellar peduncles. The brain stem should then be cut sagittally into two parts 2-mm to the right of the midline. The bigger left side should be fixed in formalin and blocked for pathologic examination. The smaller right side should be left unfixed and immediately snap-frozen for storage at −80℃. The cerebellar hemispheres should be separated sagittally through the vermis. The right cerebellar hemisphere should be dissected parasagittally into three slabs and snap-frozen. The left hemisphere should be reserved and fixed in formalin. The left cerebral hemisphere, left cerebellar hemisphere, and left-side brainstem should be fixed in 10–20% neutral buffered formalin (pH 7.4) for 2–4 weeks before tissue blocks are prepared.
The unfixed cerebral hemisphere should be dissected immediately into slabs following the same steps as described for the fixed hemisphere. First, the hemisphere should be cut coronally into two parts at the level of the mammillary bodies. The sectioning should be performed gently with one stroke per sagittal cut along an axis perpendicular to a ventrodorsal line that connects the temporal pole and occipital pole.2 The anterior part should then be dissected into 1-cm-thick slabs forwardly and numbered “S, minus, and sequential number” from the mammillary bodies throughout the frontal pole; for example, S-1, S-2, S-3, and so on. The posterior portion should also be dissected into 1-cm-thick slabs backwardly and numbered “S, plus, and sequential number” from S0, S+1, S+2, and so on. Unfixed slabs should be placed on metal trays covered with powdered dry ice with their anterior surface facing down. They should then be wrapped in aluminum foil, individually put into labeled airtight storage bags, and stored in a −80℃ freezer until investigational use. Frozen brain parenchyma aliquots when fresh or from stored frozen tissue on request for disbursement later are optional.2
Block preparation following standard section list
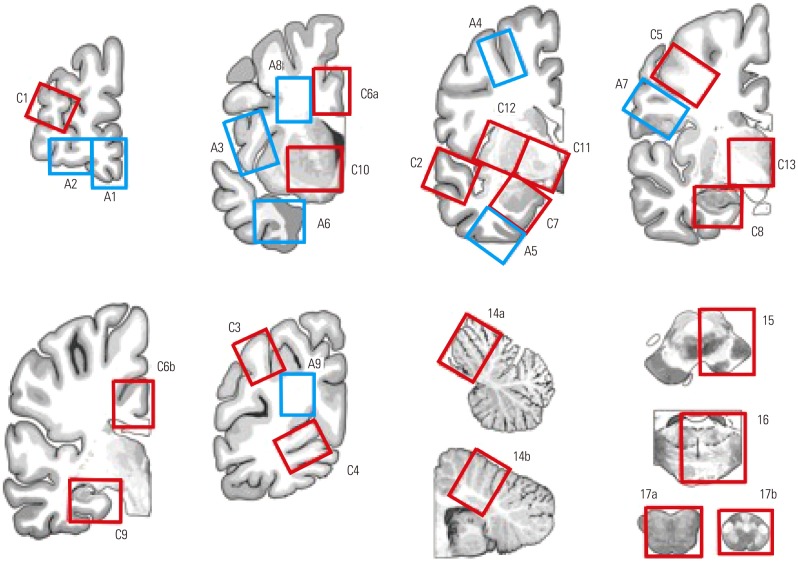
After sufficient fixation, the left hemisphere should be dissected into 1-cm-thick slabs, and blocks are prepared according to the proposed standard set of brain regions (Table 1, Fig. 1). By sharing the standard set of brain blocks, information on stored brains can easily be accessed by researchers through the data network hub. Two groups of block sites are listed in Table 1. Core blocks are crucial for pathologic confirmation of neurodegenerative diseases. Additional blocks are optional and should be based on the academic interests of pathologists or researchers. In these guidelines, we defined core blocks and additional blocks using previously published data. The recent National Institute on Aging-Alzheimer's Association (NIA-AA) revision of criteria for the pathologic diagnosis of AD proposed a minimum set of 13 histologic sections for evaluation of major neurodegenerative diseases.5,6 In addition to the minimum set of 13 regions, brain section lists were obtained from the brain donation and neuropathology manual of the Alzheimer's Disease Neuroimaging Initiative-Neuropathology Core (ADNI-NPC) at Washington University (kindly provided by Dr. Cairns), the neuropathology section list from the University of California San Francisco (kindly provided by Dr. Seeley), and the neuropathology core manual from Northwestern Memorial Hospital (kindly provided by Dr. Bigio).
Table 1. Block Sampling Sites.
| Group 1. Core blocks |
| C1. Middle frontal gyrus (BA8–9) |
| C2. Posterior superior temporal gyrus (BA22, Wernicke’s area) and middle temporal gyrus (BA21) |
| C3. Inferior parietal lobe (BA39–40, angular gyrus and supramarginal gyrus, part of Wernicke‘s area) |
| C4. Occipital lobe (BA17, calcarine sulcus) |
| C5. Precentral gyrus (BA4, primary motor cortex) |
| C6. Anterior cingulate gyrus at the level of the genu of the corpus callosum (BA24) and posterior cingulate gyrus at the level of the splenium (BA23) |
| C7. Amygdala |
| C8. Anterior hippocampus and entorhinal cortex (BA28) |
| C9. Posterior hippocampus and parahippocampal gyrus at the level of the lateral geniculate nucleus |
| C10. Ventral striatum (caudate nucleus and putamen) at the level of the nucleus accumbens |
| C11. Globus pallidus and nucleus basalis of Meynert at the level of the anterior commissure |
| C12. Insular and putamen |
| C13. Thalamus and subthalamic nucleus |
| C14. Cerebellum with dentate nucleus and vermis |
| C15. Midbrain with substantia nigra |
| C16. Pons with locus coeruleus |
| C17. Medulla oblongata with dorsal motor nucleus of the vagus and hypoglossal nucleus and upper cervical cord |
| C18. Cervical spinal cord* |
| C19. Thoracic spinal cord* |
| C20. Lumbar spinal cord* |
| Group 2. Additional blocks according to research interests |
| A1. Medial frontal pole |
| A2. Anterior orbital gyrus |
| A3. Inferior frontal gyrus, opercular part (BA44, part of Broca‘s area) |
| A4. Superior frontal sulcus |
| A5. Inferior temporal gyrus (BA20) |
| A6. Temporal pole |
| A7. Postcentral gyrus (BA3, 1, 2, primary somatosensory cortex) |
| A8. Frontal white matter |
| A9. Occipital white matter |
| A10. Olfactory bulbs |
BA, Brodmann area.
*Cut transversely at 0.5 to 2.0 cm intervals. These blocks are collected in extended autopsy with spinal cord removal.
Fig. 1. Illustration showing where to collect the core and additional blocks listed in Table 1.
Consistently identifying the central sulcus from the dissected slabs is important when blocking the motor cortex and sensory cortex. The precentral gyrus should be inked as needed. The blocks should be post-fixed for an additional 2–4 days and then processed in an automatic tissue processor. The tissue processing program should be adjusted to increase the time in ethanol tanks in order to allow sufficient dehydration of brain tissues.
Gross examination
A careful gross examination can provide a good amount of useful information for pathologic diagnosis. Vascular pathology, including stenosis or atherosclerosis, can be estimated semiquantitatively. Regional atrophy should be assessed using a semi-quantitative method involving a four-tier system of none, mild, moderate, and severe. Atrophy of the hippocampus, caudate, subthalamic nucleus, brainstem, cerebellum, and neocortices must be documented. Pallor of the substantia nigra and locus ceoruleus should be recorded. Unbalanced pallor in the substantia nigra and locus ceoruleus can help a conjectural diagnosis; AD is favored with greater pallor in the locus ceoruleus, and the opposite is seen in frontotemporal lobar degeneration (FTLD).1 Furthermore, the degree of degeneration in the cerebellar dentate nucleus and color changes of basal ganglia should be recorded.
Microscopic workup using immunohistochemical and special stains
The three most frequently encountered neurodegenerative disorders are AD, dementia with Lewy bodies (DLB) or Lewy body disease (LBD), and FTLD.1 Elements of pathologic diagnosis of neurodegenerative diseases in our guidelines are based on recent guidelines released by the NIA-AA.5 The guidelines summarize remarkable advances made since publication of the NIA/Reagan Institute of the AA Consensus Recommendations for the Postmortem Diagnosis of AD or NIA-Reagan Criteria in 1997.7
Alzheimer's disease neuropathologic change (ADNC) should be evaluated with an “ABC” score along with Aβ (β-amyloid) plaque score based on Thal phases, Braak neurofibrillary tangle (NFT) stage, and Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuritic plaque score.5,6 Immunohistochemistry (IHC)-based analysis of Aβ should be used to assess Thal phases based on progressive Aβ deposition.8 IHC staining for phospho-tau can be used to assess NFTs.8,9,10 The scope of tau pathology observed in AD includes pretangles, neurophil threads in neuronal processes, and dystrophic neuritis in neuritic plaques, as well as NFTs in cell bodies.10 Bielschowsky or Gallyas silver or thioflavin-S fluorescent special staining can be performed for differential detection of neuritic plaques from Aβ deposits.11,12 By combining ABC scores, ADNC can be transformed into one of four levels: not, low, intermediate, or high.6
LBD is a neuropathological term that encompasses the two clinical entities of Parkinson disease and DLB.13 Currently, LBD is classified as follows: brainstem-predominant, limbic (transitional), neocortical (diffuse), or amygdala-predominant.5,14 Assessment of LBD pathology includes identification of Lewy bodies on H&E staining, mainly in neurons of the brainstem sections. IHC staining for α-synuclein is the preferred method because of its high sensitivity for revealing Lewy body pathology, including Lewy neurites and variable neuronal perikaryal inclusions that comprise the continuum of immunoreactive pathology leading to Lewy body formation.13 α-synuclein IHC staining should be performed on amygdala and anterior cingulate sections. If α-synuclein pathology is identified in the anterior cingulate, additional stains can be performed on neocortical sections to determine the stage of LBD pathology.1,15
To explore FTLD pathology, tau, ubiquitin/p62, and transactive response DNA-binding protein 43 (TDP-43) staining of hippocampal and neocortex sections should be performed. To specifically examine FTLD-tau, such as corticobasal degeneration, progressive supranuclear palsy, and Pick disease, tau immunostaining should be performed on the neocortex, basal ganglia, brainstem, and dentate gyrus to determine the pathognominic inclusions of each entity.1 IHC for 3-repeat and 4-repeat tau can be beneficial in some cases. To diagnose FTLD-TDP, FTLD-fused in sarcoma (FUS), or FTLD-ubiquitin-proteasome system (UPS), a set of immunostains of corresponding antibodies should be performed on the hippocampus, cerebellum, neocortex, and deep nuclei. For diagnostic details of a wider pathologic spectrum, please refer to previously published articles.16,17 To evaluate amyotrophic lateral sclerosis, additional TDP-43 staining can be performed on spinal cord sections and medulla with hypoglossal and vagus nuclei as well as the motor cortex. Commonly used antibodies and manufacturer information are listed in Table 2. Basic stain sets for screening and an extended set for specific diagnosis are summarized in Table 3.
Table 2. Antibodies Commonly Used in the Diagnosis of Major Neurodegenerative Disorders.
| Antibody | Clone | Manufacturer | Catalogue no. |
|---|---|---|---|
| Tau | AT8 | Thermo Fisher | MN1020 |
| Beta amyloid | 4G8 | Covance | SIG-39220 |
| Phospho TDP-43 | pS409/410-2 | CosmoBio | TIP-PTD-P02 |
| Alpha-synuclein | phospho S129 | Abcam | ab59264 |
| p62 (SQSTM1) | 2C11 | Abnova | H00008878-M01 |
| Beta amyloid (1−40) | IBL | 18580 | |
| Beta amyloid (1−42) | IBL | 18582 | |
| 3-R Tau (RD3) | 8E6/C11 | Merck Millipore | 05-803 |
| 4-R Tau | CosmoBio | TIP-4RT-P01 | |
| FUS | polyclonal | Sigma-Aldrich | HPA008784 |
| Ubiquitin | DAKO | Z 0458 |
Dr. Bigio at Northwestern.
Table 3. Special and Immunohistochemical Stain Sets for Screening and an Extended Set for Specific Diagnosis.
| Site | Sliver | Aβ | Tau | α-Syn | p62/Ubiquitin | TDP-43 | |||
|---|---|---|---|---|---|---|---|---|---|
| Screen | FTLD-tau | Screen | DLB | Screen | FTLD-TDP/ALS | ||||
| MFG | V | V | V | V | V | V | V | ||
| STG | V | V | V | ||||||
| IP | V | V | V | ||||||
| Hippo | V | V | V | V | V | V | V | ||
| Amyg | V | V | V | ||||||
| Cing | V | V | |||||||
| Motor | V | ||||||||
| BG | V | V | |||||||
| Cbll | V | V | |||||||
| Stem | V | V | |||||||
| SC | V | ||||||||
Aβ, beta amyloid; FTLD, frontotemporal lobar degeneration; α-Syn, alpha-synuclein; DLB, dementia with Lewy bodies; ALS, amyotrophic lateral sclerosis; MFG, middle frontal gyrus; STG, superior temporal gyrus; IP, inferior parietal lobe; Hippo, hippocampus; Amyg, amygdala; Cing, cingulate gyrus; Motor, motor cortex; BG, basal ganglia; Cbll, cerebellum; Stem, brainstem; SC, spinal cord.
Dr. Bigio at Northwestern.
Neurodegenerative disorders are frequently accompanied by other forms that can contribute to cognitive impairment of the affected individuals.18 Nevertheless, comparative estimation for weighing of co-existing pathologic changes in terms of contribution to cognitive impairment is rarely proposed except for AD and LBD.14 It is demanding to gauge the extent to which each disease course might have contributed to cognitive dysfunction.5 Nonetheless, all observed pathologic changes should be described with regard to disorder type and severity.
As a progression of the molecular genetic study in the neurodegenerative diseses, DNA and RNA analysis from the autopsy brain sections should be recommended.19,20,21
Cerebrovascular diseases that cause vascular brain injury are mainly atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy. Vascular brain injury manifests as hemorrhages or infarcts. Infarcts are classified according to dimensions as territorial infarcts, lacular infarcts, and microinfarcts.5 All infarcts and hemorrhages observed should be documented by description of location, size, and chronicity, as a comorbidity of other degenerative illnesses, or as an isolated lesion.
In conclusion, a nationwide brain bank system is being established in South Korea. Coordination of brain donation, processing, storage, and research steps will result in a synergistic chain that might help alleviate the burden of devastating neurodegenerative diseases.2 For efficient utilization of resources and to establish a functional and efficient system, we herein proposed tentative standard operating protocols for data systemization. We hope that our proposed guidelines will lead to constructive debate and commentary that will facilitate the development of an advanced brain bank system in Korea. We anticipate that updated protocols and procedures will be developed and shared with the medical community in the future.
ACKNOWLEDGEMENTS
This research was supported by a fund (2016-ER6202-00) by Research of Korea Centers for Disease Control and Prevention.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bigio EH. Making the diagnosis of frontotemporal lobar degeneration. Arch Pathol Lab Med. 2013;137:314–325. doi: 10.5858/arpa.2012-0075-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115:509–532. doi: 10.1007/s00401-007-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross BM, Knowler JT, McCulloch J. On the stability of messenger RNA and ribosomal RNA in the brains of control human subjects and patients with Alzheimer's disease. J Neurochem. 1992;58:1810–1819. doi: 10.1111/j.1471-4159.1992.tb10057.x. [DOI] [PubMed] [Google Scholar]
- 4.Bahn S, Augood SJ, Ryan M, Standaert DG, Starkey M, Emson PC. Gene expression profiling in the post-mortem human brain--no cause for dismay. J Chem Neuroanat. 2001;22:79–94. doi: 10.1016/s0891-0618(01)00099-0. [DOI] [PubMed] [Google Scholar]
- 5.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 9.Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, et al. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59:733–748. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 12.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 13.Lowe J, Kalaria R. Dementia. In: Love S, Perry A, Ironside J, Budka H, editors. Greenfield's neuropathology. 9th ed. London: CRC Press; 2015. pp. 858–973. [Google Scholar]
- 14.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 15.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montine TJ, Larson EB. Late-life dementias: does this unyielding global challenge require a broader view. JAMA. 2009;302:2593–2594. doi: 10.1001/jama.2009.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasel JA. Validity of nucleic acid purities monitored by 260nm/280nm absorbance ratios. Biotechniques. 1995;18:62–63. [PubMed] [Google Scholar]
- 20.Barbas CF, 3rd, Burton DR, Scott JK, Silverman GJ. Quantitation of DNA and RNA. CSH Protoc. 2007;2007:pdb.ip47. doi: 10.1101/pdb.ip47. [DOI] [PubMed] [Google Scholar]
- 21.Mueller O, Lightfoot S, Schroeder A. RNA integrity number (RIN)-standardization of RNA quality control. [accessed on 2006 January 31]. Available at: http://gene-quantification.net/RIN.pdf.