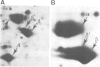
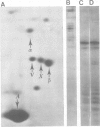
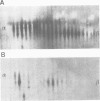
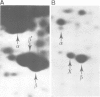
Abstract
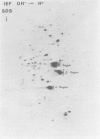
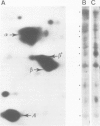
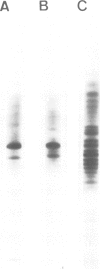
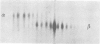
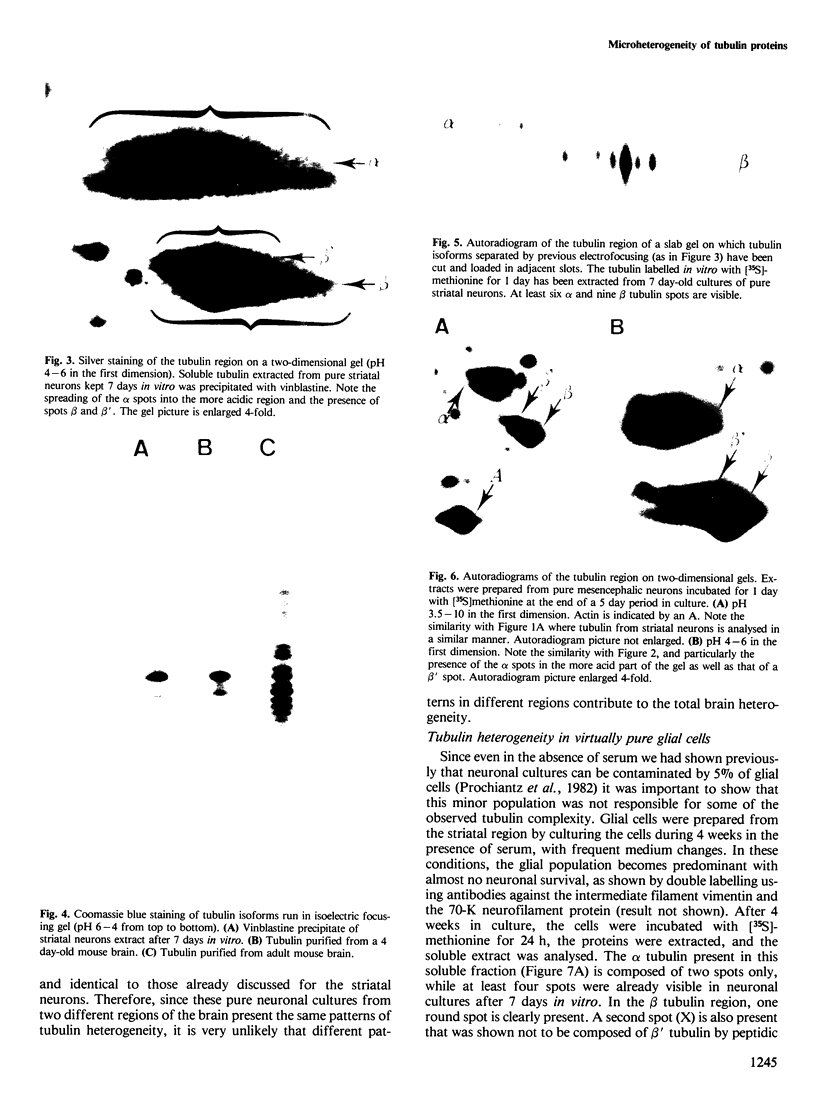
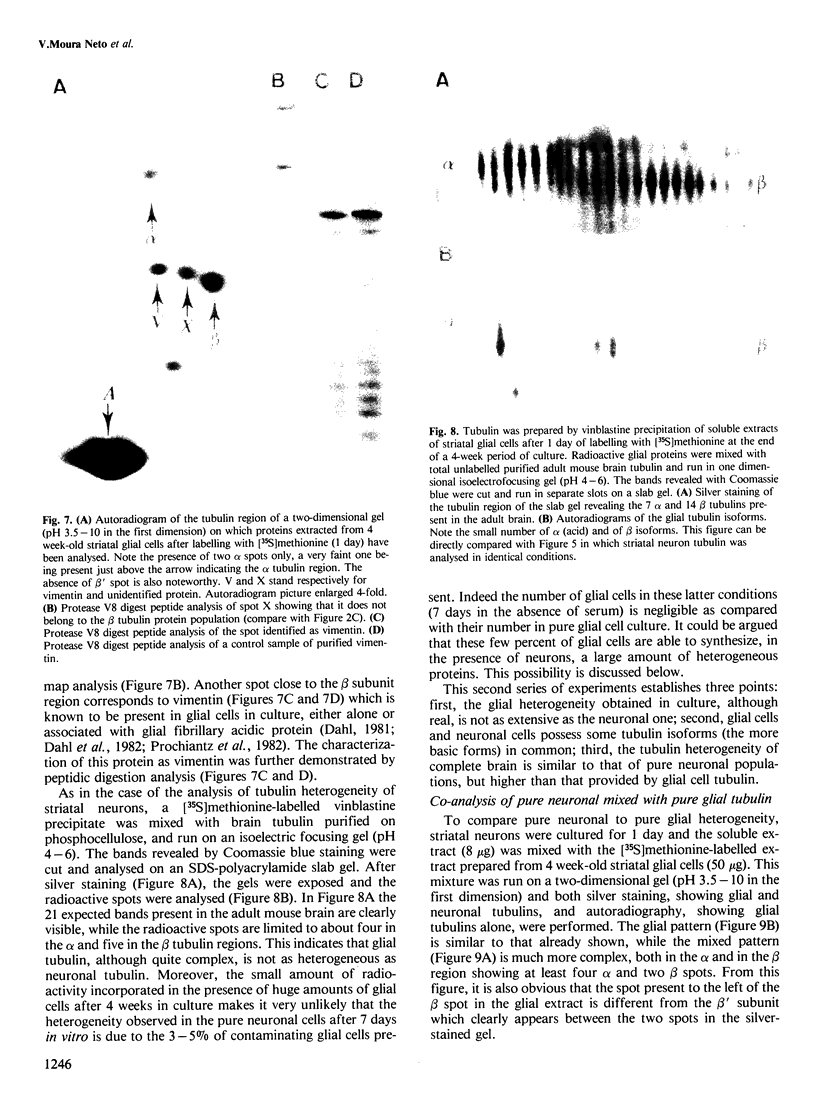
The microheterogeneity of the alpha and beta isoforms of tubulin in brain cells in culture was studied. The cells were prepared from two precise regions of the embryonic mouse brain (ED15), the striatum and the mesencephalon. It was possible to maintain virtually pure cultures of neuronal or glial cells up to 1 and 4 weeks in vitro, respectively. The tubulin heterogeneity of striatal and mesencephalic neurons was found to be very similar after a few days in culture. More precise examination of pure neurons from the striatum revealed that their tubulin content after 7 days in vitro exhibited the same degree of complexity as a control extract from a 4 day-old mouse brain. In fact, we could detect the presence of at least six alpha and nine beta tubulin isoforms. Among these isoforms a specific family of beta proteins (beta' tubulin) and the more acidic alpha proteins were present. Since these isoforms have, up to now, been found only in tubulin extracts prepared from the nervous system, our experiments suggest that they belong to the neuronal subpopulation of this tissue. This point is reinforced by their complete absence from the tubulin proteins extracted from pure glial cells even after several weeks in vitro. These results lead us to propose that brain tubulin microheterogeneity is associated with the presence of neurons and not of glia and may, therefore, play a specific role in maintaining neuronal shape and function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya B., Volff J. Membrane-bound tubulin in brain and thyroid tissue. J Biol Chem. 1975 Oct 10;250(19):7639–7646. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Nixon R. A., Marotta C. A. Posttranslational processing of alpha-tubulin during axoplasmic transport in CNS axons. J Cell Biol. 1982 Jul;94(1):159–164. doi: 10.1083/jcb.94.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski-Treska J., Guerold B., Aunis D. Immunofluorescence study on the organization of actin in astroglial cells in primary cultures. Neuroscience. 1982 Feb;7(2):509–522. doi: 10.1016/0306-4522(82)90284-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dahl D. R., Redburn D. A., Samson F. E., Jr Regional distribution of colchicine-binding (microtubular) protein in the rat brain. J Neurochem. 1970 Aug;17(8):1215–1219. doi: 10.1111/j.1471-4159.1970.tb03371.x. [DOI] [PubMed] [Google Scholar]
- Dahl D., Strocchi P., Bignami A. Vimentin in the central nervous system. A study of the mesenchymal-type intermediate filament-protein in Wallerian degeneration and in postnatal rat development by two-dimensional gel electrophoresis. Differentiation. 1982;22(3):185–190. doi: 10.1111/j.1432-0436.1982.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Dahl D. The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. J Neurosci Res. 1981;6(6):741–748. doi: 10.1002/jnr.490060608. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Weibel V. J. Changes in tubulin heterogeneity during postnatal development of rat brain. Biochem Biophys Res Commun. 1979 Feb 14;86(3):822–828. doi: 10.1016/0006-291x(79)91786-8. [DOI] [PubMed] [Google Scholar]
- Denoulet P., Edde B., Jeantet C., Gros F. Evolution of tubulin heterogeneity during mouse brain development. Biochimie. 1982 Mar;64(3):165–172. doi: 10.1016/s0300-9084(82)80466-5. [DOI] [PubMed] [Google Scholar]
- Denoulet P., Jeantet C., Gros F. Tubulin microheterogeneity during mouse liver development. Biochem Biophys Res Commun. 1982 Apr 14;105(3):806–813. doi: 10.1016/0006-291x(82)91041-5. [DOI] [PubMed] [Google Scholar]
- Edde B., Portier M. M., Sahuquillo C., Jeantet C., Gros F. Changes in some cytoskeletal proteins during neuroblastoma cell differentiation. Biochimie. 1982 Feb;64(2):141–151. doi: 10.1016/s0300-9084(82)80416-1. [DOI] [PubMed] [Google Scholar]
- Eddé B., Jeantet C., Gros F. One beta-tubulin subunit accumulates during neurite outgrowth in mouse neuroblastoma cells. Biochem Biophys Res Commun. 1981 Dec 15;103(3):1035–1043. doi: 10.1016/0006-291x(81)90913-x. [DOI] [PubMed] [Google Scholar]
- Estridge M. Polypeptides similar to the alpha and beta subunits of tubulin are exposed on the neuronal surface. Nature. 1977 Jul 7;268(5615):60–63. doi: 10.1038/268060a0. [DOI] [PubMed] [Google Scholar]
- George H. J., Misra L., Field D. J., Lee J. C. Polymorphism of brain tubulin. Biochemistry. 1981 Apr 28;20(9):2402–2409. doi: 10.1021/bi00512a006. [DOI] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., Sweadner K. J. Multiple tubulin forms are expressed by a single neurone. Nature. 1981 Dec 3;294(5840):477–480. doi: 10.1038/294477a0. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978 Oct;79(1):173–183. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Barber P. C., Sherwood M. R., Zimmer J., Raisman G. Astrocyte cultures from adult rat brain. Derivation, characterization and neurotrophic properties of pure astroglial cells from corpus callosum. Brain Res. 1982 Jul 15;243(2):329–343. doi: 10.1016/0006-8993(82)90257-8. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Harris J. L., Gilbert J. M. Characterization of multiple forms of brain tubulin subunits. J Neurochem. 1978 Jun;30(6):1431–1440. doi: 10.1111/j.1471-4159.1978.tb10475.x. [DOI] [PubMed] [Google Scholar]
- Nath J., Flavin M. A structural difference between cytoplasmic and membrane-bound tubulin of brain. FEBS Lett. 1978 Nov 15;95(2):335–338. doi: 10.1016/0014-5793(78)81024-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., Delacourte A., Daguet M. C., Paulin D. Intermediate filament proteins in mouse brain cells cultured in the presence or absence of fetal calf serum. Exp Cell Res. 1982 Jun;139(2):404–410. doi: 10.1016/0014-4827(82)90267-1. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner M., Devilliers G., Bock E., Porte A. Biochemical and ultrastructural studies of cultured rat astroglial cells: effect of brain extract and dibutyryl cyclic AMP on glial fibrillary acidic protein and glial filaments. Differentiation. 1980;17(1):51–61. doi: 10.1111/j.1432-0436.1980.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Walters B. B., Matus A. I. Tubulin in postynaptic junctional lattice. Nature. 1975 Oct 9;257(5526):496–498. doi: 10.1038/257496a0. [DOI] [PubMed] [Google Scholar]
- Wang Y. J., Mahler H. R. Topography of the synaptosomal membrane. J Cell Biol. 1976 Nov;71(2):639–658. doi: 10.1083/jcb.71.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]
- Wolff A., Denoulet P., Jeantet C. High level of tubulin microheterogeneity in the mouse brain. Neurosci Lett. 1982 Aug 31;31(3):323–328. doi: 10.1016/0304-3940(82)90041-6. [DOI] [PubMed] [Google Scholar]
- Zisapel N., Levi M., Gozes I. Tubulin: an integral protein of mammalian synaptic vesicle membranes. J Neurochem. 1980 Jan;34(1):26–32. doi: 10.1111/j.1471-4159.1980.tb04617.x. [DOI] [PubMed] [Google Scholar]
- di Porzio U., Daguet M. C., Glowinski J., Prochiantz A. Effect of striatal cells on in vitro maturation of mesencephalic dopaminergic neurones grown in serum-free conditions. Nature. 1980 Nov 27;288(5789):370–373. doi: 10.1038/288370a0. [DOI] [PubMed] [Google Scholar]
- von Hungen K., Chin R. C., Baxter C. F. Brain tubulin microheterogeneity in the mouse during development and aging. J Neurochem. 1981 Aug;37(2):511–514. doi: 10.1111/j.1471-4159.1981.tb00485.x. [DOI] [PubMed] [Google Scholar]