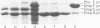Abstract
To study the role of the signal sequences in the biogenesis of outer membrane proteins, we have constructed two hybrid genes: a phoE-ompF hybrid gene, which encodes the signal sequence of outer membrane PhoE protein and the structural sequence of outer membrane OmpF protein, and a bla-phoE hybrid gene which encodes the signal sequence as well as 158 amino acids of the structural sequence of the periplasmic enzyme beta-lactamase and the complete structural sequence of PhoE protein. The products of these genes are normally transported to and assembled into the outer membrane These results show: (i) that signal sequences of exported proteins are export signals which function independently of the structural sequence, and (ii) that the information which determines the ultimate location of an outer membrane protein is located in the structural sequence of this protein, and not in the signal sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Two bacteriophages which utilize a new Escherichia coli major outer membrane protein as part of their receptor. J Bacteriol. 1978 Jul;135(1):164–170. doi: 10.1128/jb.135.1.164-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hedgpeth J., Clément J. M., Silhavy T. J., Hofnung M. Sequence analysis of mutations that prevent export of lambda receptor, an Escherichia coli outer membrane protein. Nature. 1980 May 8;285(5760):82–85. doi: 10.1038/285082a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., Verhoef C., van Alphen W. Pore protein e of the outer membrane of Escherichia coli K12. FEBS Lett. 1978 Dec 1;96(1):99–105. doi: 10.1016/0014-5793(78)81071-0. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Inokuchi K., Mizushima S. Amino acid sequence of the signal peptide of OmpF, a major outer membrane protein of Escherichia coli. FEBS Lett. 1982 Jan 25;137(2):171–174. doi: 10.1016/0014-5793(82)80341-4. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Bergmans H., van Mansfeld F., Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983 Feb 5;163(4):513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Expression of outer membrane protein e of Escherichia coli K12 by phosphate limitation. FEBS Lett. 1980 Apr 7;112(2):229–232. doi: 10.1016/0014-5793(80)80186-4. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Major outer membrane proteins of Escherichia coli strains of human origin. J Gen Microbiol. 1980 Dec;121(2):373–380. doi: 10.1099/00221287-121-2-373. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Localization of phoE, the structural gene for outer membrane protein e in Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):118–123. doi: 10.1128/jb.147.1.118-123.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Overduin P., Lugtenberg B., Bergmans H. Cloning of phoE, the structural gene for the Escherichia coli phosphate limitation-inducible outer membrane pore protein. J Bacteriol. 1982 Feb;149(2):668–672. doi: 10.1128/jb.149.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef C., de Graaff P. J., Lugtenberg E. J. Mapping of a gene for a major outer membrane protein of Escherichia coli K12 with the aid of a newly isolated bacteriophage. Mol Gen Genet. 1977 Jan 7;150(1):103–105. doi: 10.1007/BF02425330. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Control of the synthesis of alkaline phosphatase and the phosphate-binding protein in Escherichia coli. J Bacteriol. 1976 Jul;127(1):595–609. doi: 10.1128/jb.127.1.595-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hondel C. A., Keegstra W., Borrias W. E., van Arkel G. A. Homology of plasmids in strains of unicellular Cyanobacteria. Plasmid. 1979 Jul;2(3):323–333. doi: 10.1016/0147-619x(79)90016-7. [DOI] [PubMed] [Google Scholar]