Abstract
Progesterone soft capsules (brand name: Utrogestan) were demonstrated to be an effective oral alternative to prevent premature LH surges both in normal-ovulatory and polycystic ovarian syndrome (PCOS) patients. However, its safety in terms of neonatal outcomes is unclear. To evaluate whether Utrogestan use increase the risk of adverse neonatal outcomes compared with short protocol in patients undergoing IVF/ICSI treatments in combination with frozen-thawed embryo transfer (FET), we performed a retrospective analysis including 1008 FET cycles, with embryos originated from either Utrogestan + hMG protocol (n = 499), or short protocol (n = 509), which led to 546 live-born infants. The neonatal characteristics regarding preterm birth (PTB), low birth weight (LBW), gestational age and mode of delivery were comparable in the two groups. The incidence of live-birth defect was 0.68% (2/293) in the Utrogestan + hMG protocol compared with 0.79% (2/253) in the short protocol. No early neonatal death or intrauterine death were recorded in either group. To date, the data do not indicate an elevated rate of abnormality at birth after progesterone use during ovarian stimulation but further study with larger populations is needed to confirm these results.
Introduction
During the past 40 years, large scale number of infertile couples have babies through assisted reproductive technology (ART),inconsist of in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), in vitro maturation (IVM), embryo cryopreservation, frozen thawed ET, and preimplantation genetic diagnosis (PGD). ART pregnancies approximately accounts for 1.7–4% of births in developed countries, in contrast with 1.013% of births according to a report from a 2011 survey on the proportion of births born after ART in mainland China1. With the rapid development of technology and service, the proportion of ART pregnancies is steadily rising every year. As a result, the safety of ART procedures has attracted more and more attention due to the possible impact on pregnancy complications and live-birth outcomes in infants conceived by ART.
The introduction of controlled ovarian hyperstimulation (COH) has been a vital clinical milestone to increasing the success rates of ART by virtue of increasing the number of oocytes retrieved. However, premature luteinizing hormone (LH) surge was a major issue during COH, which may result in cycle cancellation owing to premature luteinization and ovulation. The usage of gonadotropin- releasing hormone agonist (GnRH-a) and GnRH antagonist has sharply reduced the occurrence of premature LH surges. More importantly, these regimens has been accepted as being safe because the incidence of obstetrical complications and congenital malformations were similar to those conceived spontaneously based on a series of follow-up studies2.
However, various disadvantages still exist, e.g., the complexity of achieving consistent downregulation, an increased risk of ovarian hyperstimulation syndrome (OHSS) from a human chorionic gonadotrophin (hCG) trigger, and expensive cost, suboptimal oocyte or embryo quality induced by premature luteinization3–5, which inspired us to explore convenient alternatives to prevent premature LH surges. When fresh embryo transfer (ET) was the norm in IVF, steroidal preparation could not be considered for use during COH because of its negative impact on endometrial receptivity though it was powerful modulators of pituitary gonadotropin (Gn) secretion6, 7. Since advanced vitrification techniques made superior quality cryopreserved embryo and precise thawing possible, frozen-thawed embryo transfer (FET) has been widely used in recent years. What’ more, the “freeze-all” strategy has been recommended due to the improved pregnancy and delivery outcomes8–10.
In combination with the “freeze-all” strategy, we corroborated luteal phase ovarian stimulation (LPS)—initiating ovarian stimulation in the luteal phase using letrozole and Gn—was able to achieve consistent LH suppression, thus, we postulated high doses of progesterone may transiently be feasible to suppress pituitary gland secretions11. Moreover, our large, retrospective study of 2,060 live-born infants found that the incidence of live-birth defects in patients undergoing COH with LPS (1.02%) was comparable with the short protocol (0.64%)12.
A hypothesis was proposed that progesterone delivered from the early follicular phase may be used to suppress premature LH surges. Soon afterwards, we verified progesterone soft capsule (brand name: Utrogestan), as a kind of natural micronized progesterone which was capable of detecting in serum after being taken orally or vaginally was an effective oral alternative for inhibiting premature LH surges both in normal-ovulatory and polycystic ovarian syndrome (PCOS) patients13–16. Furthermore, compared with the short protocol, no statistically significant differences in the pregnancy outcomes of FET were found in normal-ovulatory patients while biochemical pregnancy rate, clinical pregnancy rate, and implantation rate increased in PCOS patients undergoing COH with Utrogestan + hMG protocol13, 14. Though more studies were needed to evaluate the usage of Utrogestan from the early follicular phase, it is a new choice for patients undergoing IVF/ICSI treatments following embryo cryopreservation.
To date, hundreds of infants were born by virtue of this novel regimen. Accompanying the desire to continuously optimize this novel protocol is a concern about whether it has any adverse effect on the safety of babies and mothers. However, there are no relevant studies addressing this problem. The aim of this study was to assess neonatal outcomes after COH using Utrogestan + hMG protocol in comparison with short protocol in women undergoing IVF/ICSI treatments following a “freeze-all” strategy.
Results
The sample of 531 pregnancy cycles resulting from 1008 FET procedures, from January 1, 2014 to December 31, 2014, consisted of 441 ongoing pregnancies, 17 ectopic pregnancies, 2 heterotrophhic pregnancies, and 74 early miscarriages, resulting in a total of 546 live-born infants (432 live-birth cycles). Among them, 277 pregnancies originating from 499 FETs led to the birth of 293 live-born neonates after treatment with Utrogestan + hMG protocol group; 254 pregnancies originating from 509 FETs led to the birth of 253 live-born babies after treatment with the short protocol group (details of group distribution profiles are presented in the flow chart in Fig. 1).
Figure 1.
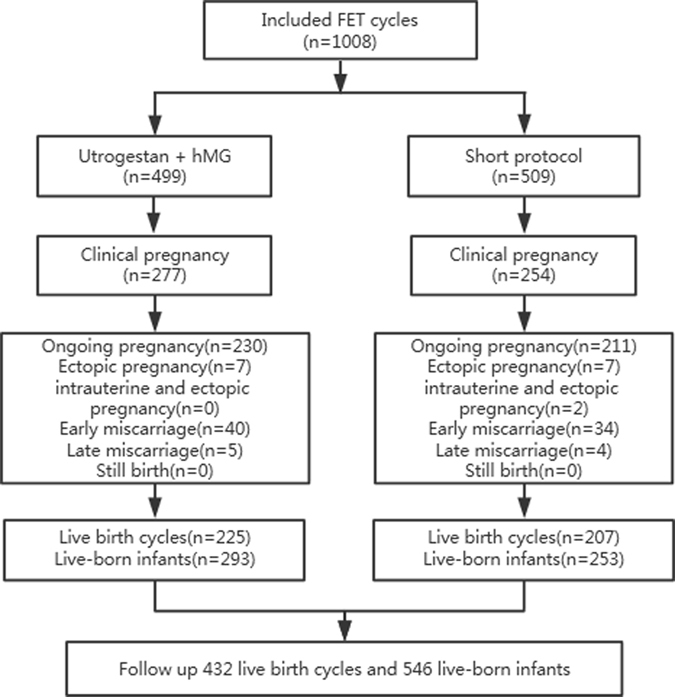
Flowchart of the study.
The characteristics of women included, such as maternal age, body mass index, infertility duration, infertility diagnosis, proportion of nullipara, endometrial preparation, and type of embryos transferred were comparable in the two groups (Table 1).
Table 1.
Baseline characteristics of sample population stratified by ovarian stimulation method.
| Outcome | Study group (Utrogestan + hMG) | Control group (Short protocol) | P |
|---|---|---|---|
| Total FETs (n) | 499 | 509 | |
| Patients (n) | 376 | 381 | |
| Nullipara | 339(90.16%) | 349(91.6%) | 0.491 |
| Maternal age (y) | 31.87 ± 4.08 | 32.11 ± 3.62 | 0.322 |
| Body mass index (kg/m2) | 21.81 ± 3.24 | 21.6 ± 2.94 | 0.189 |
| Infertility duration (y) | 3.33 ± 2.15 | 3.93 ± 2.95 | 0.346 |
| Infertility diagnosis | 0.104 | ||
| Tubal factor | 212 | 230 | |
| Male factor | 79 | 90 | |
| Combination of factors | 48 | 39 | |
| Unknown factor | 37 | 22 | |
| Endometrial preparation | 0.299 | ||
| Natural cycle | 222 | 233 | |
| Mild stimulation | 245 | 232 | |
| Hormone therapy | 32 | 44 | |
| Thawed embryos (n) | 985 | 981 | |
| Viable embryos after thawed (n) | 972 | 972 | |
| Type of embryos | 0.183 | ||
| Cleavage-stage ETs | 891 | 873 | |
| Blastocyst ETs | 81 | 99 |
Although the biochemical pregnancy rate (60.32% vs. 54.81%) and clinical pregnancy rate (55.51% vs. 49.9%) in the Utrogestan + hMG protocol group was higher than those in the short protocol group, these differences were not statistically significant (P > .05).The implantation rate (37.14% vs.34.47%), early miscarriage rate (14.44% vs. 13.39%), late miscarriage rate (1.81% vs. 1.57%), multiple pregnancy rate (32.85% vs. 31.1%), ectopic pregnancy rate (2.53% vs. 2.76%), intrauterine and ectopic pregnancy rate (0 vs. 0.79%), ongoing pregnancy rate (46.09% vs. 41.45%) were comparable in the two groups (Table 2).
Table 2.
Pregnancy and neonatal outcomes stratified by ovarian stimulation method.
| Outcomes | Study group (Utrogestan + hMG) | Control group (Short protocol) | P |
|---|---|---|---|
| Pregnancy outcomes of FET | |||
| Biochemical pregnancy rate | 60.32%(301/499) | 54.81%(279/509) | 0.077 |
| Clinical pregnancy rate per transfer | 55.51%(277/499) | 49.9%(254/509) | 0.075 |
| Implantation rate | 37.14%(361/972) | 34.47%(335/972) | 0.219 |
| Early miscarriage rate | 14.44%(40/277) | 13.39%(34/254) | 0.726 |
| Late miscarriage rate | 1.81%(5/277) | 1.57%(4/254) | 0.837 |
| Multiple pregnancy rate | 32.85%(91/277) | 31.1%(79/254) | 0.666 |
| Ectopic pregnancy rate | 2.53%(7/277) | 2.76%(7/254) | 0.869 |
| Intrauterine and ectopic pregnancy rate | 0%(0/277) | 0.79%(2/254) | 0.139 |
| Ongoing pregnant rate per transfer | 46.09%(230/499) | 41.45%(211/509) | 0.138 |
| Preterm birth rate | 17.33%(39/225) | 12.08%(25/207) | 0.124 |
| Live-birth rate per transfer | 45.09% (225/499) | 40.67%(207/509) | 0.156 |
| Neonatal outcomes | |||
| Gestational age (wk) | 0.265 | ||
| <28 | 1 | 0 | |
| 28 ≤ age < 37 | 38 | 25 | |
| ≥37 | 185 | 182 | |
| ≥42 | 1 | 0 | |
| Mode of delivery | 0.891 | ||
| Vaginal | 60 | 54 | |
| Caesarean | 165 | 153 | |
| Live born infants (n) | 293 | 253 | 0.071 |
| Single newborns (n) | 152 | 153 | |
| Single birth weight (g) | 3327.82 ± 481.4 | 3282.79 ± 520.53 | 0.559 |
| Single birth length (cm) | 49.92 ± 1.49 | 48.9 ± 6.94 | 0.902 |
| Twin newborns (n) | 146 | 108 | |
| Twin birth weight (g) | 2591.32 ± 565.99 | 2551.15 ± 463.29 | 0.98 |
| Twin birth length (cm) | 47.62 ± 6.2 | 48.04 ± 1.99 | 0.441 |
| Birth weight < 2,500 g | 26.96%(79/293) | 22.92% (58/253) | 0.278 |
| Early neonatal death | 0 | 0 | |
The live-birth rate per transfer, PTB rate, and post-term pregnancy rate in the Utrogestan + hMG protocol group (45.09%, 17.33%, and 0.44%, respectively) are not statistically different from those in the short protocol group (40.67%, 12.08%, and 0, respectively), What’s more, the mode of delivery was comparable in the two groups. Similarly, a nonsignificant difference was found among the 2 groups for the incidence of multiple delivery, despite the fact that more multiple pregnancies in the Utrogestan + hMG protocol group was deliveried than that in the short protocol group. No significant differences were found in the birth length and weight for the two groups, both in the single newborns and twins newborns. Similarly, LBW rate (22.96%vs. 22.92%) were comparable in the two groups. In addition, no early neonatal death was found in the participants (Table 2).
A total of 4 cases (0.73%) qualified as having congenital defects in all live-born infants, according to the definition in the International Classification of Diseases. Defects were observed in 2 of 293 (0.68%) in the Utrogestan + hMG protocol group, and 2 of 253 (0.79%) in the short protocol group; however, these values were all very low, and the differences was statistically significant (P > 0.05). Comparisons between groups of birth defects according to neonatal gender, singletons, and multiples were carried out and reached the undifferentiated outcomes presented in Table 3. The detailed breakdown of detected malformations according to the various organ systems is also presented in Table 3.
Table 3.
Types of congenital malformations among 546 live-born infants.
| Outcomes | Study group (Utrogestan + hMG) | Control group (Short protocol) |
|---|---|---|
| Number of birth defects | 2 | 2 |
| Number of deliveries | ||
| Singletons | 0 | 1 |
| Multiples | 2 | 1 |
| Birth defects, by gender | ||
| Male | 1 | 1 |
| Female | 1 | 1 |
| Malformation type | ||
| Circulatory system | Q21.102: atrial septal defect (1) | Q21.001: ventricular septal defect (1) |
| Digestive system | K40.903: indirect inguinal hernia (1) | / |
| Motor system | / | Q69.901: polydactyly (1) |
Discussion
Our previous studies have confirmed the oral delivery of Utrogestan is an effective way to block premature LH surges with component oocytes and embryos. However, with each new regimen, it must be shown not only that it is effective but also that it does not adversely affect the children’s health. The latter is the most important as the ultimate objective with ART is to efficiently achieve healthy live birth outcomes. The results of our study do not indicate an elevated rate of abnormality at birth after Utrogestan + hMG protocol compared with short protocol in patients undergoing IVF/ICSI treatments with FET.
Since the application of ART, concerns have been raised regarding both its safety and its effect on fetal well-being. Compared with fertile women who conceived spontaneously, a significantly higher risk of adverse obstetric outcomes such as perinatal mortality, preterm delivery (PTD), low-birth-weight (LBW), very-low-birth-weight (VLBW), and small-for-gestational-age (SGA) infants was observed in pregnancies following ART17, 18. In addition, a recent meta-analysis including birth defect information on 92 671 ART infants confirmed an increased birth defect risk exists in ART infants19. Some researchers deem the risk may be attributable to patient characteristics related to infertility, including older age, higher BMI, longer infertile time, PCOS, serious endometriosis and other related etiology of infertility20–24, while others insist it is related to the treatment-related factors, such as embryo culture conditions, cryopreservation technique, endometrial preparation, COH protocol, and other potentially relevant parameters25–28. In our study, not only the patient characteristics in terms of maternal age, body mass index, infertility duration, infertility diagnosis, proportion of nullipara were comparable, but also type of embryos transferred, the method of fertilization, endometrial preparation, luteal support and other routine laboratory procedure were similar in the two groups, thus COH protocol differs.
Compared with natural conception, COH exposure is correlated with superphysiologic steroid hormone environment may result in altered endometrial development, in consist of histologic advancement, premature down-regulation of the P receptor, an abbreviated luteal phase, glandular-stromal dyssynchrony, genomic dysregulation, altered leukocyte localization and activation, premature nucleolar channel formation, advanced angiogenesis, increased blood vessel density, and reduced endometrial blood flow26. Hu et al. corroborated that the high E2 environment resulted from COH is relevant to the increased risks of LBW and SGA. Elevated E2 level has an adverse effect on endometrial receptivity and implantation process, meanwhile, it may impair spiral artery invasion in the first trimester and compromise uteroplacental blood flow at term, leading to restricted fetal growth. Therefore, embryo cryopreservation was recommended when the maternal E2 level is extremely high on the hCG administration day and luteal support with E2 supplemention should be cautious29. The “freeze-all” strategy was indispensable because the usage of Utrogestan from the early follicular phase will affect endometrial development.
In addition, it was substantiated the degree of histologic advancement increased when P levels are prematurely elevated30. There has been contradictory results in previous literatures that pertain to the effects of P on the outcomes of IVF/ICSI. The occurrence of a preovulatory increase in serum P levels was reported to 12.4–52.3% in down-regulation protocol31. Some studies have concluded that P elevation has an detrimental effect on the pregnancy and implantation rate in patients undergoing fresh ET following GnRH agonist and antagonist cycles32, 33, whereas others claimed exert no impact on pregnancy outcomes34, 35. A better outcome was even observed in patients with PCOS or donor oocyte36, 37. Adverse effects on IVF outcomes due to elevated P level may be associated with its effect on oocyte, embryo quality and/or endometrial receptivity. A recent meta-analysis including more than 60,000 cycles conducted by Venetis et al. confirmed the main deleterious effect of P elevation acts on endometrial receptivity38. In accordance with a functional genomics analysis study, P elevation was proved to impose a detrimental effect on endometrial gene expression39, 40. The adverse impact of the high progesterone level on the endometrium can be neglected as all patients underwent FET in our study.
It was known that early embryonic survival, the establishment and maintenance of pregnancy, and even growth abnormalities is closely related to oocyte quality41. Thus, whether the administration of Utrogestan from the early follicular phase had detrimental effect on oocyte or embryo quality was a major concern. The effects of progesterone on oocyte maturation and embryo development was unclear both in vitro and in vivo. In the investigation performed by Salehnia et al., where different progesterone concentrations (10, 38, 50, 100 μM) was added to the in vitro oocyte maturation (IVM) media of mouse GV oocytes, the maturation rate decreased in a dose dependent manner and the GV arrested rates increased42. Similar reduction of oocyte maturation rate was observed in the experiment made by Fukui et al. when supplemented progesterone to IVM culture systems of bovine43. Silva et al. found that the rate of blastocyst formation was sharply reduced by the exposure of bovine cumulus oocyte complexes to progesterone44. In contrast, no significant differences was found in canine oocyte maturation among the four groups with distinct hormonal environment45. Carter et al. have shown that addition of progesterone to culture medium did not affect the proportion of in vitro matured/in vitro fertilized zygotes that developed to the blastocyst stage in vitro 46. In addition, it is intriguing that better results was obtained with the exposure of progesterone. Supplementation of canine oocyte culture media with progesterone and E2 was found to stimulate oocyte maturation47, 48. Zhang and Armstrong reported that the addition of progesterone to porcine oocyte maturation medium could improve both fertilization and cleavage rates49. Furthermore, it was validated the high progesterone concentration in follicular fluid were associated with better embryo development in humans and rhesus monkeys50. Our previous research confirmed this possibility to some extent, as the viable embryo rate per oocyte retrieved was significantly improved both in nomal-ovulatory patients and PCOS patients using Utrogestan + hMG protocol.
The serum P values increased swiftly after Utrogestan administered orally, with a range of 0.9–47.8 ng/mL reported in our previous studies, and then maintained at stable concentrations following continuous delivery. The absorption and elimination of Utrogestan were dose independent, thus a considerable inter-individual variation was recognized16. Though it was shown that oocytes aspirated during the luteal phase, a physiological context of high progesterone with an average peak level of 11.1 ng/mL, are capable of maturing in vitro and being fertilized11, no powerful evidence has proven the safety of high progesterone exposure in the early follicular phase for offspring that originate from this ovarian-stimulation regimen. Therefore, we followed up on the live-birth defects of infants born from Utrogestan + hMG protocol. In our study, the clinical pregnancy rate, implantation rate, ongoing pregnancy rate, live birth rate were comparable in the two groups, which illustrated that the high context of progesterone did not impair the pregnancy outcomes. Furthermore, various birth characteristics in consist of weeks of gestation, PTB rate, postterm delivery rate, birth weight and length, odds of LBW, multiple delivery rate, early neonatal mortality, and incidence of live-birth defects, were similar in the two groups. The results of this study indicated that the artificial high context of progesterone by taking Utrogestan was not associated with any significant increase in risk of pregnancy or neonatal outcomes compared with short protocol in patients undergoing IVF/ICSI treatments in combination with FET.
The incidence of live-birth defects was not higher, compared with the rate in those using the traditional ovarian stimulation regimen. The total rate of congenital malformation in live-born infants was 0.73% in our study, slightly lower than the 1.09% rate for abnormalities occurring in infants within 7 days after delivery, in People’s Republic of China, as reported in another study51. In fact, the study indicated that Utrogestan + hMG protocol is safe for live-born infants at birth. The reason why the rate of birth defects was lower in our study can be elucidated from the following aspects. First and foremost, women with maternal diseases or adverse environmental exposure during pregnancy was excluded from the study. In addition, all the participants underwent FET, which have been reported to have a lower risk of birth defects compared with fresh ET cycles52. Furthermore, induced abortion because of detectable malformations were excluded from the study. Fetal deformities tend to be timely diagnosed and terminated due to patients’ heightened awareness and concern in an IVF-conceived pregnancy.
A major limitation of our study is the retrospective design of this study and small sample size. Although no significant differences were found between the two groups in the rate of congenital anomalies, this may have been due to a type II error in view of the small sample size. Additionally, the data about the neonatal information were collected from parent questionnaires, rather than from direct access to medical records. However, the findings offer an insight into the safety of the use of Utrogestan for ovarian stimulation in combination with FET.
In conclusion, our preliminary study found that the data to date do not indicate an elevated rate of adverse neonatal outcomes after ovarian stimulation using Utrogestan + hMG protocol, but further study with larger populations is needed to confirm this finding. Application of Utrogestan for the prevention of premature LH surges has advantages of oral administration, user convenience, fee reduction and increased user compliance. These results will help the popularization and application of the new regimen as an oral alternative to GnRH analogue treatment, in combination with embryo cryopreservation.
Materials and Methods
Study Population and Design
A retrospective cohort study was conducted at the Department of Assisted Reproduction of the Ninth People’s Hospital of Shanghai Jiao Tong University School of Medicine (Shanghai, People’s Republic of China). The study was approved by the hospital’s ethics committee. Infertile couples, who underwent IVF or ICSI treatments with frozen-thawed embryo transfer (FET) using Utrogestan + hMG protocol, or the standard GnRH-a short protocol, were recruited at our center. Informed written consent was obtained from patients in accordance with the ethics committee protocol. The procedure about collecting neonatal outcome data was was extensively described elsewhere12. Briefly, a telephone interview with couples was the primary way, and family planning service agencies were contacted when connection with couples was lost.
These patients underwent the FET procedures from January 1, 2014 to December 31, 2014. Infants born to mothers with reported maternal diseases, such as gestational diabetes mellitus, hypertension, and thyroid disorders, or adverse environmental exposure during pregnancy, were excluded from this analysis because of the possible association of these factors with birth defects. The final data, involving 546 live-born infants, were stratified into groups according to the protocol of ovarian stimulation: 293 births after Utrogestan + hMG protocol and 253 births after the standard GnRH-a short protocol. The study design and participant selection procedure are presented in Fig. 1.
Treatment
HMG 150 to 225 IU (Maanshan Pharmaceutical Trading Co., Anhui, China) and Utrogestan (Laboratories Besins International, Paris, France) 100 mg twice a day were administered from MC3 until the trigger day. In the study group After 6 to 7 days, follicular monitoring was performed using a transvaginal ultrasound examination to record the number of developing follicles,while serum FSH, LH, E2, andprogesterone concentrations were measured. When 3 dominant follicles reached 18mm in diameter, the final stage of oocyte maturation was triggered using triptorelin 0.1 mg (Decapeptyl, Ferring Pharmaceuticals, Germany). Transvaginal ultrasound-guided oocyte retrieval was conducted 34 to 36 hours after the trigger. All follicles with diameters of more than 10 mm were retrieved.
In the control group,triptorelin 0.1 mg daily beginning on MC2 and hMG 150 to 225 IU daily beginning on MC3 were administered. The ultrasound examination was started at MC9–11 and repeated every 2–4 days. The serum hormone level tests were performed on the same days as the ultrasound exams. The hMG dose was adjusted according to the follicle development and the serum hormone levels. When the dominant follicles reached 18mm in diameter, the final stage of oocyte maturation was induced with an intramuscular injection of hCG 3000 IU. Oocyte retrieval was conducted 34 to 36 hours later.
Fertilization of the aspirated oocytes was performed by either IVF or ICSI, depending on the semen parameters. According to the number and regularity of blastomeres and the degree of embryonic fragmentation, good-quality embryos (including grade 1 and grade 2, 8-cell embryos) were frozen by vitrification on the third day following the oocyte retrieval, and non-top-quality embryos were placed in extended culture, of which good morphological grade blastocysts were frozen on day 5 or day 6.
The method of embryo and endometrium synchronization during a natural cycle, an induced ovulation cycle, or an artificial cycle; and the timing of ET are extensively described elsewhere (11–13). Once pregnancy was achieved, the progesterone supplement was continued until 10 weeks of gestation.
Pregnancy and neonatal outcomes
Pregnancy outcomes included clinical pregnancy rate, early miscarriage rate, late miscarriage rate, implantation rate, ongoing pregnancy rate, ectopic pregnancy rate and live birth rate. Clinical pregnancy was defined as the presence of a gestational sac with fetal heart activity during ultrasound examination 7 weeks after FET. The early miscarriage rate was defined as the proportion of patients with spontaneous pregnancy termination before the gestational age of 12 weeks. The late miscarriage rate was defined as the proportion of patients with pregnancy termination after the gestational age of 12 weeks. The multiple pregnancy rate was defined as the number of patients with two or more gestational sacs divided by the number of pregnant patients. The implantation rate was defined as the number of gestational sacs divided by the number of embryos transferred. The live-birth rate was defined as the proportion of patients with live birth among all transfer cycles.
Neonatal outcomes included preterm birth (PTB), low birth weight (LBW), gestational age, mode of delivery, early neonatal mortality and congenital malformations.
PTB was defined as deliveries before 37 weeks of gestation. LBW was defined as birth weights within 2500 g. Early neonatal death was defined as the death of a live-born baby within 7 days of birth. Congenital anomalies were defined as all structural, functional, and genetic anomalies diagnosed in aborted fetuses, at birth or in the neonatal period. Congenital malformations were classified using the International Classification of Diseases Q codes (Q00–Q99) as conditions registered in the International Statistical Classification of Diseases and Related Health Problems12.
Statistical Analysis
Statistical analyses were carried out using SPSS 19.0 software (SPSS, Inc.). Data were presented as means ± SD; qualitative data were presented as percentages. Data were analyzed using the Student’s t-test, Mann-Whitney U-test, and, x2-test where appropriate. The Mann-Whitney U-test was used for the variables of non-normal distribution. P < 0.05 was considered statistically significant.
Acknowledgements
This study was funded by the National Nature Science Foundation of China (grant number: 81601344) and the Natural Science Foundation of Shanghai (17411963700).
Author Contributions
H.J.Y. collected the data; X.X.Z. designed the study and wrote the first draft of the manuscript; Y.l.F. selected studies for inclusion and revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang X, Li Y, Li C, Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014;101:385–391. doi: 10.1016/j.fertnstert.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Bonduelle M, Oberyé J, Mannaerts B, Devroey P. Large prospective, pregnancy and infant follow-up trial assures the health of 1000 fetuses conceived after treatment with the GnRH antagonist ganirelix during controlled ovarian stimulation. Hum Reprod. 2010;25:1433–1440. doi: 10.1093/humrep/deq072. [DOI] [PubMed] [Google Scholar]
- 3.van Loenen AC, Huirne JA, Schats R, Hompes PG, Lambalk CB. GnRH agonists, antagonists, and assisted conception. Semin Reprod Med. 2002;20:349–364. doi: 10.1055/s-2002-36713. [DOI] [PubMed] [Google Scholar]
- 4.Bosch E, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80:1444–1449. doi: 10.1016/j.fertnstert.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 6.Cedrin-Durnerin I, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum Reprod. 2007;22:109–116. doi: 10.1093/humrep/del340. [DOI] [PubMed] [Google Scholar]
- 7.Letterie GS. Inhibition of gonadotropin surge by a brief mid-cycle regimen of ethinyl estradiol and norethindrone: possible role in in vitro fertilization. Gynecol Endocrinol. 2000;14:1–4. doi: 10.3109/09513590009167652. [DOI] [PubMed] [Google Scholar]
- 8.Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102:19–26. doi: 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, et al. Is frozen embryo transfer cycle associated with a significantly lower incidence of ectopic pregnancy? An analysis of more than 30,000 cycles. Fertil Steril. 2014;102:1345–1349. doi: 10.1016/j.fertnstert.2014.07.1245. [DOI] [PubMed] [Google Scholar]
- 10.Roque M, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Kuang Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101:105–111. doi: 10.1016/j.fertnstert.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103:1194–1201. doi: 10.1016/j.fertnstert.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XX, Zhang XL, Fu YL. Utrogestan as an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Medicine (Baltimore). 2015;94:e909. doi: 10.1097/MD.0000000000000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu XX, Zhang XL, Fu YL. Effect of progesterone used to prevent LH surges in controlled ovarian stimulation. Reprod Contra. 2015;35:384–388. [Google Scholar]
- 15.Zhu XX, Ye HJ, Fu YL. The Utrogestan and hMG protocol in patients with polycystic ovarian syndrome undergoing controlled ovarian hyperstimulation during IVF/ICSI treatments. Medicine (Baltimore). 2016;95:e4193. doi: 10.1097/MD.0000000000004193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu, X. X., Ye, H. J. and Fu, Y. L. The use of Utrogestan during controlled ovarian hyperstimulation in normally ovulating women undergoing in vitro fertilization or intracytoplasmic sperm injection treatments in combination with a “freeze all” strategy: a randomized controlled dose-finding study of 100 mg versus 200 mg. Fertil Steril. (Epub ahead of print) (2016). [DOI] [PubMed]
- 17.Pinborg A, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 18.McDonald SD, et al. Preterm birth and low birth weight among in vitro fertilization twins: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2010;148:105–113. doi: 10.1016/j.ejogrb.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19:330–353. doi: 10.1093/humupd/dmt006. [DOI] [PubMed] [Google Scholar]
- 20.Jackson S, et al. Pregnancy outcomes in very advanced maternal age pregnancies: the impact of assisted reproductive technology. Fertil Steril. 2015;103:76–80. doi: 10.1016/j.fertnstert.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong, X. et al. Effect of parental physiological conditions and assisted reproductive technologies on the pregnancy and birth outcomes in infertile patients. Oncotarget. (Epub ahead of print) (2016). [DOI] [PMC free article] [PubMed]
- 22.Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovarian syndrome. Semin Reprod Med. 2008;26:72–84. doi: 10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, et al. Effects of female and male body mass indices on the treatment outcomes and neonatal birth weights associated with in vitro fertilization/intracytoplasmic sperm injection treatment in China. Fertil Steril. 2016;106:460–466. doi: 10.1016/j.fertnstert.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Wennberg AL, et al. Effect of maternal age on maternal and neonatal outcomes after assisted reproductive technology. Fertil Steril. 2016;106:1142–1149. doi: 10.1016/j.fertnstert.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Sunkara SK, La Marca A, Seed PT, Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod. 2015;30:1473–1480. doi: 10.1093/humrep/dev076. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro BS, Daneshmand ST, Bedient CE, Garner FC. Comparison of birth weights in patients randomly assigned to fresh or frozen-thawed embryo transfer. Fertil Steril. 2016;106:317–321. doi: 10.1016/j.fertnstert.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 27.Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: a controlled national cohort study. Hum Reprod. 2013;28:230–240. doi: 10.1093/humrep/des377. [DOI] [PubMed] [Google Scholar]
- 28.Huang B, et al. Neonatal outcomes after early rescue intracytoplasmic sperm injection: an analysis of a 5-year period. Fertil Steril. 2015;103:1432–1437. doi: 10.1016/j.fertnstert.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Hu XL, et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab. 2014;99:2217–2224. doi: 10.1210/jc.2013-3362. [DOI] [PubMed] [Google Scholar]
- 30.Venetis CA, et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3296 IVF cycles. Hum Reprod. 2015;30:684–691. doi: 10.1093/humrep/deu362. [DOI] [PubMed] [Google Scholar]
- 31.Venetis CA, et al. Is P elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007;13:343–355. doi: 10.1093/humupd/dmm007. [DOI] [PubMed] [Google Scholar]
- 32.Elgindy EA. Progesterone level and progesterone/estradiol ratio on the day of hCG administration: detrimental cutoff levels and new treatment strategy. Fertil Steril. 2011;95:1639–1644. doi: 10.1016/j.fertnstert.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 33.Bosch E, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–2100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 34.Seow KM, et al. Subtle progesterone rise in the single-dose gonadotropin-releasing hormone antagonist (cetrorelix) stimulation protocol in patients undergoing in vitro fertilization or intracytoplasmic sperm injection cycles. Gynecol Endocrinol. 2007;23:338–342. doi: 10.1080/09513590701403629. [DOI] [PubMed] [Google Scholar]
- 35.Saleh HA, Omran MSEA, Draz M. Does subtle progesterone rise on the day of HCG affect pregnancy rate in long agonist ICSI cycles? J Assist Reprod Genet. 2009;26:239–242. doi: 10.1007/s10815-009-9309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Pregnancy: premature luteinization as detected by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte in-vitro fertilization. Hum Reprod. 1993;8:1506–1511. doi: 10.1093/oxfordjournals.humrep.a138288. [DOI] [PubMed] [Google Scholar]
- 37.Doldi N, et al. Elevated serum progesterone on the day of HCG administration in IVF is associated with a higher pregnancy rate in polycystic ovary syndrome. Hum Reprod. 1999;14:601–605. doi: 10.1093/humrep/14.3.601. [DOI] [PubMed] [Google Scholar]
- 38.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability or pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19:433–457. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 39.Labarta E, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813–1825. doi: 10.1093/humrep/der126. [DOI] [PubMed] [Google Scholar]
- 40.Van V, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online. 2011;22:263–271. doi: 10.1016/j.rbmo.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82:E14–23. doi: 10.2527/2004.8213_supplE14x. [DOI] [PubMed] [Google Scholar]
- 42.Salehnia M. Progesterone shifts the pinopodes expression of mouse endometrium to pre-implantation time after ovarian hyperstimulation. Iran J Reprod Med. 2003;1:20–23. [Google Scholar]
- 43.Fukui Y, Fukushima M, Terawaki Y, Ono H. Effect of gonadotropins, steroids and culture media on bovine oocyte maturation in vitro. Theriogenology. 1982;18:161–175. doi: 10.1016/0093-691X(82)90100-5. [DOI] [PubMed] [Google Scholar]
- 44.Silva CC, Knight PG. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil. 2000;119:261–269. doi: 10.1530/reprod/119.2.261. [DOI] [PubMed] [Google Scholar]
- 45.Hewitt DA, England GC. Effect of preovulatory endocrine events upon maturation of oocytes of domestic bitches. J Reprod Fertil Suppl. 1997;51:83–91. [PubMed] [Google Scholar]
- 46.Carter F, et al. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol Reprod. 2010;83:707–719. doi: 10.1095/biolreprod.109.082354. [DOI] [PubMed] [Google Scholar]
- 47.Willingham-Rocky LA, Hinrichs K, Westhusin ME, Kraemer DC. Effects of stage of oestrous cycle and progesterone supplementation during culture on maturation of canine oocytes in vitro. Reproduction. 2003;126:501–508. doi: 10.1530/rep.0.1260501. [DOI] [PubMed] [Google Scholar]
- 48.Vannucchi CI, de Oliveira CM, Marques MG, Assumpcao ME, Visintin JA. In vitro canine oocyte nuclear maturation in homologous oviductal cell co-culture with hormone- supplemented media. Theriogenology. 2006;66:1677–1681. doi: 10.1016/j.theriogenology.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Armstrong DT. Effects of follicle-stimulating hormone and ovarian steroids during in vitro meiotic maturation on fertilization of rat oocytes. Gamete Res. 1989;23:267–277. doi: 10.1002/mrd.1120230304. [DOI] [PubMed] [Google Scholar]
- 50.Morgan PM, Boatman DE, Bavister BD. Relationships between follicular fluid steroid hormone concentrations, oocyte maturity, in vitro fertilization and embryonic development in the rhesus monkey. Mol Reprod Dev. 1990;27:145–151. doi: 10.1002/mrd.1080270209. [DOI] [PubMed] [Google Scholar]
- 51.Yan J, et al. Birth defects after assisted reproductive technologies in China: analysis of 15,405 offspring in seven centers (2004 to 2008) Fertil Steril. 2011;95:458–460. doi: 10.1016/j.fertnstert.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Halliday JL, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25:59–65. doi: 10.1093/humrep/dep364. [DOI] [PubMed] [Google Scholar]


