Abstract
Low-density neutrophils (LDNs) are a subset of neutrophils first described in the bloodstream upon pathological conditions, and recently, in the blood of healthy humans. LDNs may have an enhanced pro-inflammatory (low-density granulocytes, LDGs) or an immunosuppressive (Granulocytic myeloid-derived suppressor cells, G-MDSCs) profile. Whether these characteristics are specific to LDNs or related to disease states is unknown. Thus, we sought to investigate the properties of LDNs in both health and disease states, and to compare them to those of autologous normal-density neutrophils (NDNs). We studied 8 horses with severe equine asthma and 11 healthy animals. LDNs were smaller and contained more N-formylmethionine-leucyl-phenylalanine receptors than NDNs, but the myeloperoxidase content was similar in both cell populations. They also had an increased capacity to produce neutrophil extracellular traps, and were more sensitive to activation by phorbol-12-myristate-13-acetate. This profile is suggestive of LDGs. These characteristics were similar in both healthy and diseased animals, indicating that these are intrinsic properties of LDNs. Furthermore, these results suggest that LDNs represent a population of primed and predominantly mature cells. This study is the first to characterize LDNs in health, and to compare their properties with those of NDNs and of animals with a naturally occurring disease.
Introduction
Neutrophils are key players in the inflammatory response, and they are the first leukocytes to reach tissues to fight against infectious agents and various other aggressors1. They were initially considered terminally differentiated cells2, but it is now recognized that neutrophils are a heterogeneous cell population, composed of subsets displaying distinct properties3, 4. Low-density neutrophils (LDNs) are neutrophils that co-segregate with blood mononuclear cells after density-gradient separation techniques. They have been reported to be present in the bloodstream of human patients suffering from autoimmune disorders (e.g. systemic lupus erythematosus or SLE)5–9, cancer10, 11, systemic and local infection12–17, dermatomyelosis18, malaria19 and asthma20. LDNs have also been observed in the peripheral blood of pigs after experimental viral infection21 and of rats with pristane-induced arthritis22, but have not yet been reported in the blood of animals during naturally occurring disease processes.
Several findings in humans suggested that LDNs can display an enhanced pro-inflammatory profile with an increased synthesis of cytokines (TNF-α, IL-6/-8, IFN type I)8, capable of contributing to neutrophilic recruitment and persistence in chronic inflammatory conditions. They also have an increased proclivity to spontaneously produce neutrophil extracellular traps (NETs)5, in a process known as NETosis23, and circulating LDN levels are correlated with disease state and severity in humans14, 20, 24. Because of these findings, LDNs were initially considered as an aberrant, pathological population of cells8, 24, and the term “low-density granulocytes” (LDGs) has been introduced to describe LDNs with pro-inflammatory properties. Since then, however, LDNs were reported to also be present in the bloodstream of healthy human subjects14, 20, 25 suggesting that similarly to NDNs, they are a normal cell population that may be increased in number under disease conditions.
Because of their expression of defensin9 and their morphology7, it has been postulated that LDNs are immature cells, progenitors of normal-density neutrophils (NDNs) that are prematurely released from the bone marrow secondary to an increased recruitment during inflammation26. Conversely, it has been suggested that LDNs are mature NDNs activated following inflammatory signals26 or that they derived from progenitor cells distinct from those leading to NDNs6. However, it was also proposed that they were a mixed population8 or even, mostly mature cells12. Clearly, the presence of LDNs in the blood of healthy individuals, their maturation status, their origin, and their enhanced pro-inflammatory profile compared to NDNs, remain controversial. Therefore, the present study was performed to evaluate the properties of LDNs in health and during chronic asthmatic inflammation. We hypothesized that LDNs have several characteristics that may not be influenced by the health status of the subject. We first determined that LDNs were present in the blood of healthy horses and of animals affected with severe equine asthma (heaves), a neutrophilic inflammatory airway disease commonly affecting adult horses27. We then characterized these cells (morphologically, phenotypically and functionally) both in healthy and diseased horses.
Results
Quantification of Low-Density Neutrophils in the PBMCs
LDNs were identified in the peripheral blood mononuclear cell (PBMC) layer from both healthy and asthmatic horses, and levels were not affected by the age and the sex of the animals. Horses with severe asthma in exacerbation of the disease had a significantly greater percentage (35.9% ± 7.13) and absolute number (1.05 × 106 ± 2.89 × 105 cells per ml) of LDNs in the PBMC layer compared with controls (7.0% ± 0.62, p = 0.05 and 2.48 × 105 ± 5.19 × 104 cells per ml, p = 0.03, respectively; Fig. 1A,B). The percentages of LDNs decreased during disease remission in 5 of the 6 asthmatic horses when compared to disease exacerbation, but this difference was not statistically significant (p = 0.11, Fig. 1C).
Figure 1.
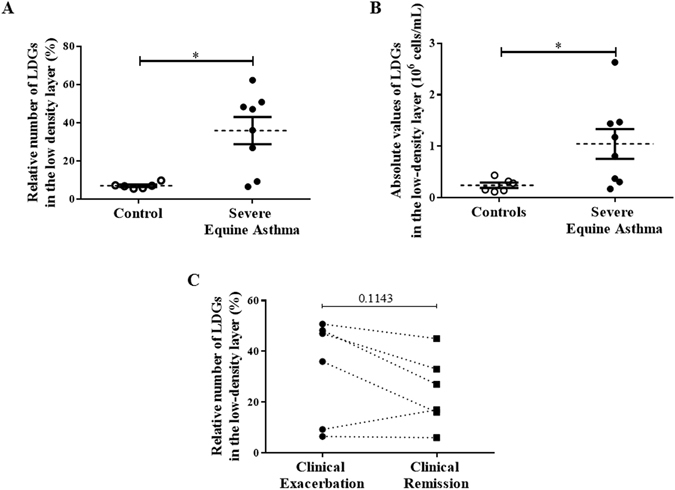
Levels of low-density neutrophils (LDNs). (A,B) Percentages and numbers of LDNs in peripheral blood mononuclear cells (PBMCs) of controls and horses with severe equine asthma during disease exacerbation. Each symbol denotes a single animal, and the mean ± SEM for each study population is shown. *p < 0.05 compared with control. (C) Percentages of LDNs in PBMCs of horses with severe equine asthma, comparing clinical exacerbation with clinical remission (p = 0.1143). Each symbol denotes a single animal.
There was a significant increase of the NDN absolute number in severe equine asthma (3.94 × 106 ± 5.91 × 105) when compared to controls (2.63 × 106 ± 3.05 × 105, p = 0.04), but the values remained within the normal range for this species28. There were no other significant differences in the numbers of cells isolated from each layer in all groups (data not shown). Eosinophils were only found in the normal density layer. The values remained within the normal range for horses28 and no difference between control and asthmatic horses (4.73 × 105 ± 1.13 × 105 and 5.34 × 105 ± 1.10 × 105, respectively; data not shown) were observed.
Morphological evaluation
Morphological evaluation was performed to assess the maturity of LDNs. Immature granulocytes were considered as having a hyposegmented nucleus with 2 lobes or less, but also a greater diameter (Fig. 2A)29, 30. In each group of horses, there was had significantly less LDNs with a normally segmented nucleus (88.7% ± 2.93 in control horses and 89.9% ± 2.53 in asthmatic horses) compared to NDNs (98.0% ± 0.59 in control horses, p = 0.03 and 96.5% ± 0.36 in asthmatic horses, p = 0.05; Fig. 2B).
Figure 2.
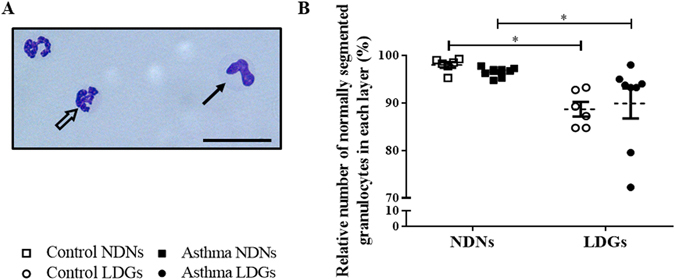
Levels of normally segmented granulocytes in each layer. (A) Representative photography of cytospins (x400, stained Protocol Hema 3) of the peripheral blood mononuclear cell layers (scale bar = 28 µm). Mature granulocytes (empty arrow) have more than 2 nuclear lobes (classically between 3 and 4) connected by filaments, whereas immature granulocytes (full arrow) have a curved nucleus with 2 or fewer nuclear lobes. LDNs were quantitated morphologically by light microscope. (B) Percentages of normally segmented LDNs in peripheral blood mononuclear cells of controls and horses with severe asthma. Each symbol denotes a single animal (mean ± SEM). *p < 0.05 compared with control.
In both control and asthmatic horses, LDNs were significantly smaller (10.82 µm ± 0.22, and 10.98 ± 0.16 µm, respectively; Fig. 3) than NDNs (12.10 µm ± 0.34, p = 0.006 and 12.82 ± 0.22 µm, p < 0.0001, respectively). There was no significant effect of the condition on the segmentation of the nucleus, nor on the cell diameter in either type of granulocytes. However, there was a trend for NDNs from asthmatic horses to be bigger than those of controls (p = 0.08).
Figure 3.
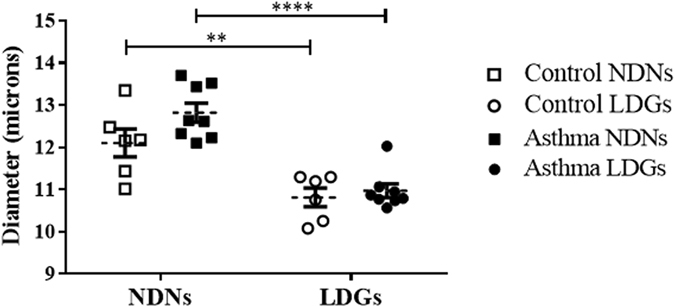
Size of Low-Density Neutrophils (LDNs) and Normal-Density Neutrophils (NDNs). Each symbol denotes the size (diameter) mean for a single horse, and the mean ± SEM for each studied population is shown. **p ≤ 0.01 and ****p ≤ 0.0001.
Flow cytometry
The intracellular levels of myeloperoxidase (MPO) have been used to evaluate the maturity of neutrophils and to determine if the cells had degranulated8. LDNs and NDNs displayed comparable levels of intracellular MPO expression (Fig. 4) in the present study and it was not affected by the health status of the animals.
Figure 4.
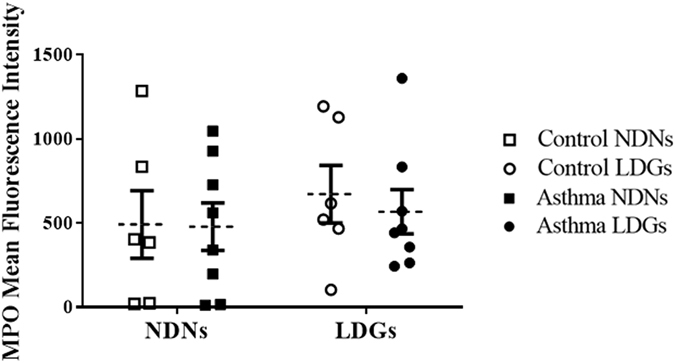
Mean Fluorescence Intensity of MPO in both layers of cells. Each symbol denotes the size mean for a single animal. The mean ± SEM for each studied population is shown.
Immunofluorescence
The expression of the N-formylmethionine-leucyl-phenylalanine receptor (fMLP-R) in the granules was measured as a marker for neutrophil maturity, as it has been shown to increases in mature in neutrophils9, 31. In this study, fMLP-R signal appeared as red at the immunofluorescence and the lobularity of the nuclei was used in order to identify the granulocytes (Fig. 5A). Significantly more LDNs expressed the fMLP-R (69.9% ± 4.22) when compared to NDNs (21.0% ± 6.79, p < 0.0001; Fig. 5B). While severe equine asthma had no impact on the expression of fMLP-R in LDNs, they were significantly increased in asthmatic NDNs compared to those from healthy horses (31.6% ± 10.6 and 6.83% ± 1.40, respectively; p = 0.05; Fig. 5B).
Figure 5.
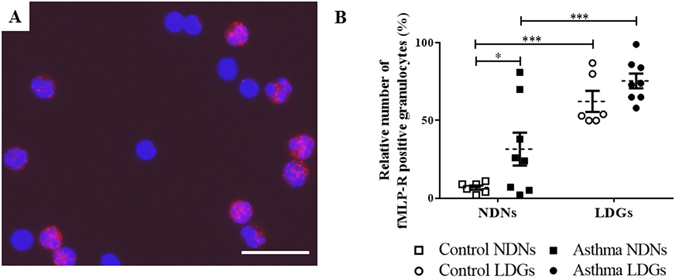
Immunofluorescence of fMLP-R in both layers of cells. (A) Representative photography at ×400 magnification (scale bar = 28 µm) of using an Axio Imager M.1 microscope (Zeiss) of the low-density layer. fMLP-R appeared as red points by immunofluorescence, giving the cells containing this receptor a piknotic aspect. The DNA appears in blue. (B) Percentages of low-density neutrophils and normal-density neutrophils positive for fMLP-R. Each symbol denotes a single animal. Mean ± SEM for each studied population is shown. *p ≤ 0.05 and ***p ≤ 0.001.
NET production
NETs on confocal microscopy appear as a thin white filament originating from a nucleus and orientating toward another one (Fig. 6A). Spontaneous NET formation was enhanced in LDNs when compared to NDNs in both group of horses (Fig. 6B). After logarithm transformation, the mean NET area per neutrophil in control horses for LDNs and NDNs was −4.50 ± 0.07 log of µm²/neutrophil and −5.14 ± 0.14 (p = 0.03), respectively. In asthmatic horses, the values for LDNs and NDNs were −4.88 ± 0.12 and −5.57 ± 0.19, respectively (p = 0.02).
Figure 6.
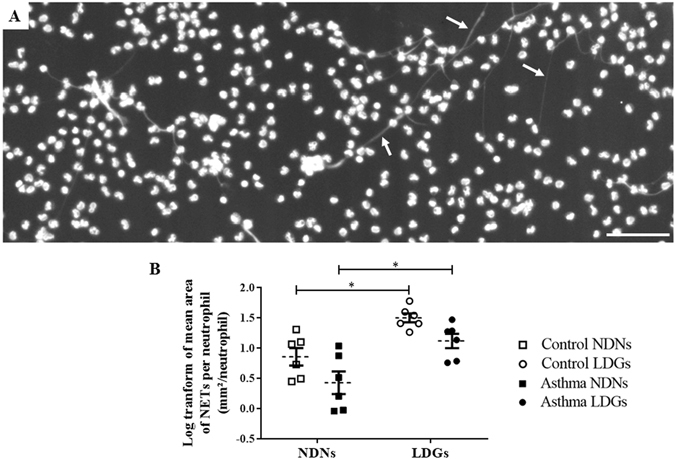
Neutrophil extracellular traps (NETs) production in both layers of cells. (A) Representative photography of using a MRC1024 confocal laser-scanning microscope at ×100 magnification (BioRad, Hercules, CA) equipped with a Nikon Eclipse TE300 camera (Nikon, Tokyo, Japan) of the low-density layer (scale bar = 100 µm). White arrows indicate NETs’ structures. (B) Log transform of the mean area of NETs per neutrophil in each layer (non-stimulated NS). Each symbol denotes a single animal. Mean ± SEM for each studied population is shown. *p ≤ 0.05.
Stimulation with phorbol-12-myristate-13-acetate (PMA) significantly increased the production of NETs by NDNs and LDNs in both groups of horses (p = 0.0008, Fig. 7A). Fold increases also indicated that LDNs produced significantly more NETs than NDNs (between 2.40 to 4.70 more, p < 0.001, Fig. 7B). When expressed as LDN/NDN ratios, NET mean area per neutrophil was significantly increased only in non-stimulated LDNs of control horses (3.70-fold increase and p = 0.03 without PMA, against 2.40-fold increase and p = 0.56 with PMA). However, asthmatic LDNs produced significantly more NETs with and without PMA (4.70-fold increase and p = 0.001 for non-stimulated LDNs, against a 4.60-fold increase and p = 0.002 for stimulated LDNs).
Figure 7.
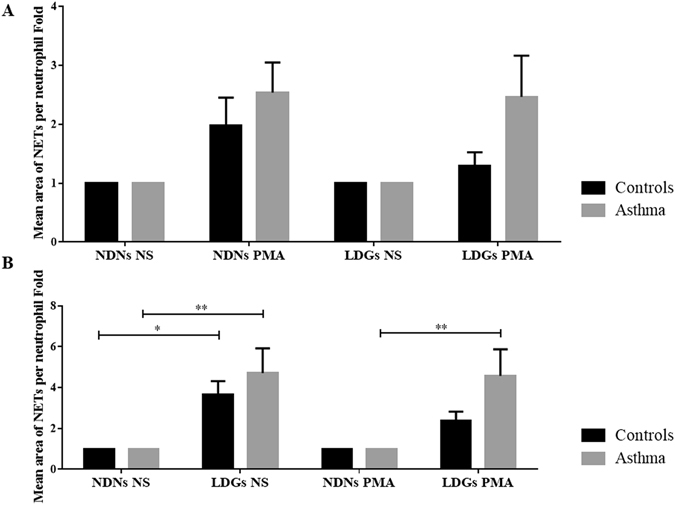
Fold changes of mean area of NETs per neutrophil in each layer of cells. (A) Results represent fold induction of the effects of PMA stimulation on the mean area of NETs per neutrophil on each layer of cells in each group of horses. Stimulation with PMA significantly increased the production of NETs in both groups of horses (p = 0.0008). Mean ± SEM for each studied population is shown. A two-way repeated measures ANOVA without Sidak’s multiple comparison post-tests has been realized in this case. (B) Results represent fold induction of the mean area of NETs per neutrophil by LDNs compared to NDNs in each group of horses, before and after stimulation with PMA. LDNs produced significantly more NETs than NDNs (p < 0.001). Mean ± SEM for each studied population is shown. *p ≤ 0.05 and **p ≤ 0.01.
Discussion
LDNs are now recognized as a subset of neutrophils that may be found in the blood of human patients in association with disease severity in various inflammatory conditions. Whether the low buoyancy of these cells results from degranulation of NDNs, or from a distinct property or maturation process is unclear, as their contribution to disease processes. The present study provides new insights into several aspects of LDNs during asthmatic inflammation, but also importantly, in health. LDNs were present in the bloodstream of healthy horses and their levels were increased in asthmatic animals, as observed in humans20. The LDNs levels in the blood of asthmatic horses decreased during clinical remission of the disease, but remained above those of healthy controls. Moreover, compared to autologous NDNs, LDNs exhibited morphological, phenotypical and functional differences that were present in both healthy and asthmatic horses. These results suggest that LDNs have intrinsic properties that are neither influenced nor secondary to asthma, but that these cells increase in number and may be primed during inflammatory states.
LDNs have intrinsic properties
Several differences between LDNs and NDNs were observed in the present study. LDNs were smaller than NDNs, which might contribute to their low buoyancy. Phenotypically, LDNs also had a different granular content (more fMLP-R) compared to NDNs and they had an increased capacity to produce NETs. These differences were present in both healthy and asthmatic horses, suggesting that these properties may be intrinsic to LDNs. fMLP-R are chemoattractant receptors that belong to the G protein-coupled receptor family32. When activated by N-formyl peptides such as N-formylmethionine-leucyl-phenylalanine (fMLP), they contribute to the physiological defense against bacterial infection and cell disruption33. This increased fMLP-R in LDNs suggest that these cells are more sensitive to activation stimuli and is in agreement with their proposed enhanced pro-inflammatory profile8 and anti-bacterial activities.
LDNs are present in the bloodstream of healthy horses but there was a mean 5-fold increase in numbers in the blood of asthmatic horses, as reported in human asthma20. The percentages of LDNs decreased, although not significantly, during disease remission when compared to exacerbation in asthmatic horses, and remained above values of controls, indicating that levels of LDNs vary with the severity of the disease. This is in agreement with the association between LDNs and human asthma severity20 and the possible role of the LDNs as a clinical biomarker. The lack of significant difference between these two disease states is likely explained by the low power of the study for this effect (it was estimated that 2 additional asthmatic horses would have been necessary in order to have 80% of chances to observe a significant difference). Also, a longer period of antigen avoidance (6 weeks in the present study) may have resulted in a significant decrease of the LDNs when compared to exacerbation state or even to a complete resolution of the asthmatic inflammation (LDN values similar to control horses). Indeed, in patients with pulmonary tuberculosis, LDN levels required 2 weeks of medical treatment to significantly decreased, and a 6-month period to be equal to those of healthy volunteers12.
LDNs are a mixed population of immature and mature cells
LDNs were first considered as immature cells because of their low buoyancy7, 9, 13, their elevated expression of the cluster of differentiation 33 (CD33)14, and their granulopoiesis signature9. In the present study, the receptor for the chemoattractant fMLP was used to assess maturity as it is synthesized in the final stage of the maturation of the neutrophils34. The increased expression of fMLP-R in LDNs we observed at the proteomic level is in agreement with the increased mRNA expression found in SLE children9. Associated with the decreased diameter and the segmentation of the nuclei30, with the lack of difference of the MPO content we observed, these findings suggest that LDNs are mostly a mature population of neutrophils, unlike what was initially suggested18, 26. LDNs were also reported as mature neutrophils based on the surface molecular expression (CD10, CD15, CD16, CD66b, CD11b), although some cells had hyposegmented nuclei (band cells, lobular nuclei) rather indicating immaturity8, 9, 20, 21.
NET production
Their increased formation of NETs in the present study suggests that LDNs in equine asthma have pro-inflammatory properties and may then belong to the group of LDGs. Furthermore, it suggest that they are more sensitive to activation stimuli compared to NDNs. NETs are chromatin filaments released by neutrophils that are associated with nuclear, cytoplasmic and granular proteins23. They have a function in host defense by protecting against pathogens and may cause direct epithelial and endothelial cell damages, by releasing toxic proteins (such as MPO)35 and by exposing autoantigens36. Two studies in humans5, 37 have also reported that unstimulated LDNs undergo significantly more NET formation compared to NDNs. In severe equine asthma, but not in healthy horses, unstimulated and stimulated LDNs produced almost the same amount of NETs and they were more sensitive to the stimulation compared to NDNs. This is in agreement with the results of Villanueva et al.5 in SLE patients, and support the hypothesis that these cells are primed in the diseased subjects24.
Several hypotheses regarding LDN origin have been suggested. An unidentified stimulus may alter the normal development of neutrophils in the bone marrow (e.g. early release, altered gene expression)22, 26 or may act directly on mature circulating neutrophils resulting in their lower buoyancy (e.g. activation, degranulation)12, 26. Another hypothesis proposes that LDNs and NDNs originate from different progenitor cells6. The evaluation of their surface markers in SLE patients indicated that LDNs may display some characteristics of activated neutrophils. However, other indexes (L-selectin shedding, levels of MPO and of ROS, transmission electron microscopy) rather suggested that they are not an activated and degranulated subset of neutrophils8, 26. Our results with MPO mean fluorescence intensity would be in agreement with this latter observation. However, the increased NETs formation and fMLP-R production suggest that LDGs may nevertheless be more easily activated than NDNs30. It is also consistent with their enhanced pro-inflammatory profiles in asthma as formylated peptides are well-known pro-inflammatory molecules38, 39.
Horses and severe equine asthma as a model for LDN study
Severe equine asthma is a spontaneously and commonly occurring disease of adult horses, associated with bronchospasm, mucus accumulation and remodeling of the airways leading to periods of dyspnea40. Airway neutrophilia is a characteristic finding of this condition, with these cells infiltrating the lungs of susceptible horses as early as 5–6 h after antigen exposure, and preceding the development of airway obstruction41. The increased circulating LDNs we observed in asthmatic horses indicates that they possibly contribute to the disease expression, by enhanced NET production. The presence of NETs in the lungs of asthmatic horses but not in controls has been reported42.
Equine asthma is not only a disease of veterinary importance, but it is also considered as a suitable model for human asthma27, 40, because of the numerous similarities between the conditions. Furthermore, equine and human neutrophils have similar biology30, 43–46 and the remodeling of asthmatic airways affects the epithelium, extracellular matrix and smooth muscle layers in both species40. Results of the current study also indicate that horses are an appropriate model to study unresolved issues regarding the origin or the pathophysiology of LDNs in health, and their contribution to neutrophilic asthma. The size of horses facilitates these studies, as it allows collecting non-invasively large amount of blood (and cells) without altering the animal immune response and measuring various physiological parameters (bronchoalveolar lavage fluid or BALF, respiratory mechanics, and lungs biopsies), without anesthesia, or scarifying the animals, as in rodents.
In conclusion, results of the present study suggest that LDNs in equine asthma are a population of mostly mature and primed cells, having characteristics that are distinct from those of NDNs, in both health and disease. According to the criteria enunciated by the Scapini et al.3, 47, it appears that LDNs in asthma have pro-inflammatory properties and are then LDGs. Our study also highlights the possible contribution of LDNs to domestic animal diseases and the suitability of horses as a model for the study of LDNs in human asthma. The presence of LDNs in healthy patients suggests that they could be a physiologic subset of neutrophils with a purpose in the homeostasis of the organism and that their increased expression in some disease cause a dysregulation contributing to the pathogenesis. However, more data are required before to assess this hypothesis.
Material and Methods
Experimental design
Study 1
14 horses (6 healthy and 8 asthmatic) were stabled and fed hay for at least 30 days to cause exacerbation of asthma in susceptible animals. The amount of circulating LDNs, and the morphological (diameter and segmentation of nuclei) and phenotypical (flow cytometry and immunofluorescence) evaluations of neutrophils (LDNs and NDNs) were studied. The amount of circulating LDNs was also evaluated in 6 of these asthmatic horses while at pasture for 6 weeks to induce clinical remission of the disease.
Study 2
The production of NETs by LDNs and NDNs was assessed in 12 horses (6 healthy and 6 asthmatic) stabled and fed hay for at least 30 days.
Animals
Eight mixed-breed adult horses with severe asthma (means of 527.6 ± 16.3 kg and 15.1 ± 1.78 years of age, mean ± SEM) and 11 age-matched healthy controls (means of 512.7 ± 7.41 kg and 12.4 ± 1.16 years of age) from the research herd of the Equine Asthma Research Laboratory at the Université de Montréal (including 16 mares and 3 geldings) were studied. The two groups of horses were housed together during the entire course of the study. Horses with severe equine asthma had a previous history of airway obstruction documented by lung function measurements and pulmonary neutrophilia in BALF (≥25%) upon stabling and hay feeding48. Control horses had no history or clinical signs suggesting airway diseases. The degree of respiratory impairment in horses were assessed daily by clinical scoring49, 50. A score from 0 to 4 is attributed to nasal flaring (0: no flaring; 4: severe, continuous flaring during each respiration) and abdominal movement (0: no abdominal effort; 4: severe, marked abdominal movement). Both scores are added for a maximal score of 8. Scores ≥4 indicates respiratory dysfunction. Furthermore, at the beginning of the study and at the time of the sampling, respiratory mechanics were performed using an impulse oscillometry (IOS) device as described by Van Erck et al.51 with the Equine MasterScreen IOS system (Jaeger, Würzburg, Germany). However, these data are not presented in this paper because part of another study conducted by Fillion-Bertrand et al. (paper submitted) at the same time than our and including the same horses. All experimental procedures were performed in accordance with the guidelines of the Canadian Council for Animal Care and were approved by the Animal Care Committee of the Faculty of Veterinary Medicine of the Université de Montréal (Rech-1716).
Neutrophil isolation
Blood was drawn by venipuncture in a jugular vein using sterile heparinized tubes (Tyco healthcare, Pointe-Claire, QC, Canada). NDNs and peripheral blood mononuclear cells (PBMCs) were isolated according to the manufacturer’s instructions. Briefly, after 30 to 45 minutes of sedimentation, the plasma-rich layer was recovered and used in a density gradient centrifugation method with Ficoll-PaqueTM Premium 1084 (GE Healthcare Bio-sciences Corp, Mississauga Canada). Five ml of the PBMCs layer was harvested (Fig. 8) and the NDN layer was collected in the bottom of the tubes after erythrocyte lysis using a hypotonic treatment with distilled water (Thermo Fisher Scientific, Burlington, ON, Canada). Cells were washed and suspended in a buffer solution containing phosphate-buffered saline (PBS) 1X, EDTA 0.5 mM (Thermo Fisher Scientific), and BSA 0.2% (Sigma-Aldrich, St Louis, MO, USA). Cell counting and viability were evaluated using ADAM automatic Cell Counter (Montreal-Biotech Inc., Montréal, QC, Canada). The viability of NDNs and PMBCs were 98.23 ± 0.22% (mean ± SEM), and 98.63 ± 0.33%, respectively.
Figure 8.
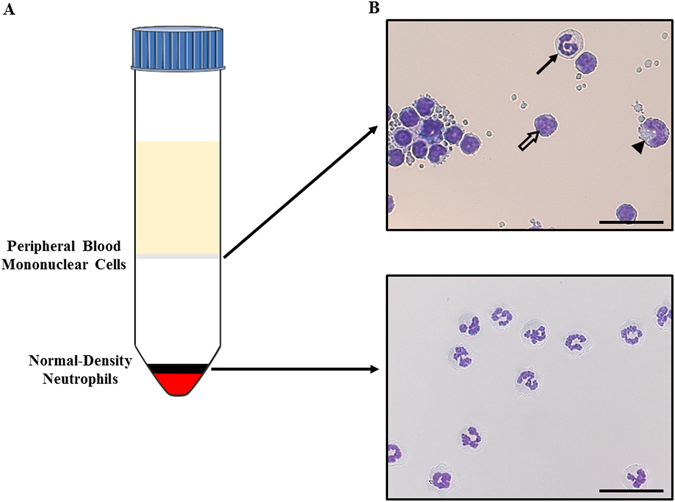
Isolation procedure for the neutrophil subsets from equine blood. (A) Ficoll density gradient separating Low-Density Neutrophils (migrating at the interface blood/gradient together with peripheral mononuclear cells) from Normal-Density Neutrophils migrating at the bottom of the gradient together with red blood cells. (B) Neutrophil subsets morphology evaluated by optical microscopy at 400× magnification (scale bar = 28 µm). Upper panel: LDNs (full arrow) with mononuclear cells (lymphocytes indicated by the empty arrow and monocytes indicated by the arrowhead). Lower panel: NDNs after erythrolysis by a hypotonic treatment. Neutrophil subsets were cytocentrifuged and stained with Protocol Hema 3 and imaged using the NanoZoomer 2.0-HT system.
Cytopreparations (Cytospin, Rottorfix Hettish) were stained with Protocol Hema 3 (Fisher Canada, Nepean, Canada) and a differential count performed on 400 cells, with the assessor blinded to sample origin. The purity of NDNs was 97.7% ± 0.37. There were 5.77% ± 0.58 LDGs in PMBCs layers for the control horses and 26.81% ± 4.19 for the asthmatic horses. Cells were then fixed 20 minutes in paraformaldehyde 2%, washed three times in PBS 1X and stored in 500 µL of PBS 1X at 4 °C until analyses.
Flow cytometry
Intracellular MPO content was evaluated in each layer (NDNs and LDNs). Prior to staining, 106 cells were harvested and washed twice in PBS 1X. All antibody incubation steps were performed at room temperature.
Granulocytes and PMBCs/LDNs were resuspended in blocking buffer (PBS 1X containing 2% FBS) and incubated on ice for 20 minutes. The cells suspension was then permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) for 5 min and incubated with anti-rabbit MPO (IgG, 16 mg/L, Dako, Denmark) and with a monoclonal canine DH24A antibody52–54 (IgM, 15 µg/mL, VMRD, Pullman WA, USA) for 45 minutes in order to select equine neutrophils. After three washes in washing buffer, cells were incubated in dark for 45 min with secondary goat Alexa488-coupled anti-rabbit IgG antibodies (1:500 in washing buffer, Thermo Fisher Scientific) and goat PE anti-mouse IgM antibody (1:1000 in washing buffer, Invitrogen). Cells were then washed twice in washing buffer (PBS 1X) and suspended in 500 μL PBS before flow cytometry acquisition of 10 000 events and analysis using Cellquest Pro software on a FACScalibur instrument (BD Biosciences). Isotype-matched control antibodies (mouse IgM and rabbit IgG) were used to set photomultipliers (PTM) voltage and compensation parameters for fluorescence detection in FL-1 and FL-2 channels.
Immunofluorescence
fMLP-R expression was evaluated in each layer (NDNs and LDNs). Prior to staining, 106 cells were harvested and washed twice in PBS 1X.
Granulocytes were resuspended in blocking buffer (PBS 1X containing 2% FBS) and incubated on ice for 20 min. The cell suspension was then permeabilized with 0.3% Triton X-100 and incubated with anti-FPRL1 antibody [GM1D6] (2 mg/mL; ab26316, Abcam, Germany) for 45 min. Cells were washed three times in washing buffer and incubated in dark for 45 min with secondary goat Alexa594-coupled anti-mouse IgG antibody (1:500 in washing buffer, Invitrogen) and 50 μg/ml of 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories). Cells were then washed twice in washing buffer and suspended in 500 μL PBS 1X, for finally being mounted in a drop of ProLong Antifade reagent (P36930; Thermo Fisher Scientific).
Images were taken using an Axio Imager M.1 microscope (Zeiss) and analyzed using Zen software (Fig. 5A). A library of images was randomly established in order to have at least 200 granulocytes for each slide. Cells were identified as neutrophils based on nuclear morphology (segmentation of the nucleus) and because of the paucity in eosinophils and basophils (data not shown) by an operator blinded to horses and to the layer.
Morphological evaluation
Granulocytes (NDNs and LDNs) were classified as immature or mature according to their nuclear segmentation. Nuclei displaying >2 nuclear lobes were considered as mature, those with ≤2 lobes were classified as immature granulocytes (Fig. 2A). At least 400 granulocytes were evaluated by an assessor blinded to sample origin.
All slides were then digitized at 200× magnification with the NanoZoomer 2.0-HT system (Hamamatsu Photonics, SZK, Japan). The diameter of each type of granulocyte was measured using ImageJ (http://rsb.info.nih.gov/ij/) with cells approximated as circles. The evaluator was blinded to slide identification and measurements were over 200 randomly selected granulocytes.
Induction of NET formation and DNA staining
Neutrophils were isolated as described above except that blood was drawn in sterile EDTA tubes, as heparin dismantles NETs55. They were resuspended in complete RPMI and seeded (106) onto six-well plates containing 1.5 mm-thick poly-L-lysine-coated coverslips, stimulated for 3 h with 200 nM PMA, and fixed for 20 min in cold methanol. After three washes in PBS 1X, a DNA-staining technique is performed by incubation of cells with propidium iodide (PI; 50 μg/ml) for 5 min at room temperature, and washed three times with PBS. The coverslips were then mounted in a drop of ProLong Antifade reagent (Thermo Fisher Scientific) and images were acquired with a MRC1024 confocal laser-scanning microscope at magnification ×100 (BioRad, Hercules, CA) equipped with a Nikon Eclipse TE300 camera (Nikon, Tokyo, Japan) and a Perfect Focus System (Fig. 7A).
NET quantification
NET production was blindly assessed with NewCast software version 4.5.1.324b (Visiopharm, Denmark). A region of interest (ROI) was defined, for each image in order to exclude the border of the slides (5 mm from the border of each side). The regions where the focus prevented a reliable assessment of the cells were excluded. The NET mean area per granulocyte was assessed on 25% of the ROI (randomly selected by the software). A point counting technique using grids with 900 crosses per screen was performed (this point density allowed to reliably evaluate the structures of interest). NET mean area per granulocyte was calculated for each horse as follows: ANETs = (4*Across*ΣPNETs)/(Estimated number of granulocytes), where Across indicates the area occupied per one cross (area of the ROI divided per 900) and ΣPNETs the sum of the points falling onto a NETs. The differential count of granulocytes per layer allowed the correction of the calculated area by the number of studied cells in each image (differential * 1 × 106).
Statistical analysis
Analyses were carried out using Prism 6.05 (GraphPad Software Inc, CA, USA). For cells quantification, data were analyzed with unpaired t-tests with Welch’s correction. For all other analysis, a two-way repeated measures ANOVA with a Sidak’s multiple comparisons post-test. For NET quantification, differences between means were analyzed using t test or by a two-way repeated measures ANOVA with or without Sidak’s multiple comparison post-tests, where a p ≤ 0.05 was considered significant.
All the results are expressed regarding the following presentation: mean ± SEM.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The authors thank Dr. Carolyn Grimes and Dr. Caroline Cluzel for their expert counsel in clinical pathology, Dr. Sophie Mainguy-Seers for the help in sampling and Denis Flipo (Department of Biological Sciences, UQÀM) for the help with the confocal microscopy. This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author Contributions
N.H., A.V. and J.-P.L. wrote the main manuscript text and N.H. prepared Figs 1–8. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 4.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N, et al. Genomic alterations in abnormal neutrophils isolated from adult patients with systemic lupus erythematosus. Arthritis Res Ther. 2014;16:R165. doi: 10.1186/ar4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 8.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marini O, et al. Identification of granulocytic myeloid-derived suppressor cells (G-MDSCs) in the peripheral blood of Hodgkin and non-Hodgkin lymphoma patients. Oncotarget. 2016;7:27676–27688. doi: 10.18632/oncotarget.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagiv JY, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, et al. Low-Density Granulocytes Are Elevated in Mycobacterial Infection and Associated with the Severity of Tuberculosis. PLoS One. 2016;11:e0153567. doi: 10.1371/journal.pone.0153567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drifte G, Dunn-Siegrist I, Tissieres P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 14.Cloke T, Munder M, Taylor G, Muller I, Kropf P. Characterization of a novel population of low-density granulocytes associated with disease severity in HIV-1 infection. PLoS One. 2012;7:e48939. doi: 10.1371/journal.pone.0048939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cloke, T. et al. Phenotypic alteration of neutrophils in the blood of HIV seropositive patients. PLoS One8, e72034, doi:10.1371/journal.pone.0072034 (2013). [DOI] [PMC free article] [PubMed]
- 16.Lood C, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin AM, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, S., Shen, H., Shu, X., Peng, Q. & Wang, G. Abnormally increased low-density granulocytes in peripheral blood mononuclear cells are associated with interstitial lung disease in dermatomyositis. Mod Rheumatol, 1–8, doi:10.1080/14397595.2016.1179861 (2016). [DOI] [PubMed]
- 19.Rocha BC, et al. Type I Interferon Transcriptional Signature in Neutrophils and Low-Density Granulocytes Are Associated with Tissue Damage in Malaria. Cell Rep. 2015;13:2829–2841. doi: 10.1016/j.celrep.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu J, Tobin MC, Thomas LL. Neutrophil-like low-density granulocytes are elevated in patients with moderate to severe persistent asthma. Ann Allergy Asthma Immunol. 2014;113:635–640 e632. doi: 10.1016/j.anai.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Porntrakulpipat S, Depner KR, Moennig V. Are low-density granulocytes the major target cells of classical swine fever virus in the peripheral blood? J Vet Med B Infect Dis Vet Public Health. 2001;48:593–602. doi: 10.1046/j.1439-0450.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann MH, et al. The cathelicidins LL-37 and rCRAMP are associated with pathogenic events of arthritis in humans and rats. Ann Rheum Dis. 2013;72:1239–1248. doi: 10.1136/annrheumdis-2012-202218. [DOI] [PubMed] [Google Scholar]
- 23.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 24.Midgley A, Beresford MW. Increased expression of low density granulocytes in juvenile-onset systemic lupus erythematosus patients correlates with disease activity. Lupus. 2016;25:407–411. doi: 10.1177/0961203315608959. [DOI] [PubMed] [Google Scholar]
- 25.Ssemaganda A, et al. Characterization of neutrophil subsets in healthy human pregnancies. PLoS One. 2014;9:e85696. doi: 10.1371/journal.pone.0085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclere M, Lavoie-Lamoureux A, Lavoie JP. Heaves, an asthma-like disease of horses. Respirology. 2011;16:1027–1046. doi: 10.1111/j.1440-1843.2011.02033.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith, B. P. Large animal internal medicine. Fifth edition. edn (Elsevier Mosby, 2015).
- 29.Linderkamp O, Ruef P, Brenner B, Gulbins E, Lang F. Passive deformability of mature, immature, and active neutrophils in healthy and septicemic neonates. Pediatr Res. 1998;44:946–950. doi: 10.1203/00006450-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho LO, Aquino EN, Neves AC, Fontes W. The Neutrophil Nucleus and Its Role in Neutrophilic Function. J Cell Biochem. 2015;116:1831–1836. doi: 10.1002/jcb.25124. [DOI] [PubMed] [Google Scholar]
- 31.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur J Pharmacol. 2006;534:1–11. doi: 10.1016/j.ejphar.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol. 1999;66:989–995. doi: 10.1002/jlb.66.6.989. [DOI] [PubMed] [Google Scholar]
- 35.Saffarzadeh M, Preissner KT. Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr Opin Hematol. 2013;20:3–9. doi: 10.1097/MOH.0b013e32835a0025. [DOI] [PubMed] [Google Scholar]
- 36.Liu CL, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grayson PC, et al. Neutrophil-Related Gene Expression and Low-Density Granulocytes Associated With Disease Activity and Response to Treatment in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2015;67:1922–1932. doi: 10.1002/art.39153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender JG, McPhail LC, Van Epps DE. Exposure of human neutrophils to chemotactic factors potentiates activation of the respiratory burst enzyme. J Immunol. 1983;130:2316–2323. [PubMed] [Google Scholar]
- 39.Issekutz AC, Lee KY, Biggar WD. Enhancement of human neutrophil bactericidal activity by chemotactic factors. Infect Immun. 1979;24:295–301. doi: 10.1128/iai.24.2.295-301.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullone M, Lavoie JP. Asthma “of horses and men”-How can equine heaves help us better understand human asthma immunopathology and its functional consequences? Molecular immunology. 2014 doi: 10.1016/j.molimm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Brazil TJ, et al. Kinetics of pulmonary neutrophil recruitment and clearance in a natural and spontaneously resolving model of airway inflammation. Clin Exp Allergy. 2005;35:854–865. doi: 10.1111/j.1365-2222.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- 42.Cote O, et al. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS One. 2014;9:e96217. doi: 10.1371/journal.pone.0096217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsch G, Lavoie-Lamoureux A, Beauchamp G, Lavoie JP. Neutrophils are not less sensitive than other blood leukocytes to the genomic effects of glucocorticoids. PLoS One. 2012;7:e44606. doi: 10.1371/journal.pone.0044606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murcia RY, Vargas A, Lavoie JP. The Interleukin-17 Induced Activation and Increased Survival of Equine Neutrophils Is Insensitive to Glucocorticoids. PLoS One. 2016;11:e0154755. doi: 10.1371/journal.pone.0154755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bullone M, Moran K, Lavoie-Lamoureux A, Lavoie JP. PI3K and MAPKs regulate neutrophil migration toward the airways in heaves. J Vet Intern Med. 2013;27:164–170. doi: 10.1111/jvim.12008. [DOI] [PubMed] [Google Scholar]
- 46.Brazil TJ, Dixon PM, Haslett C, Murray J, McGorum BC. Constitutive apoptosis in equine peripheral blood neutrophils in vitro. Vet J. 2014;202:536–542. doi: 10.1016/j.tvjl.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273:48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- 48.Couetil LL, et al. Inflammatory Airway Disease of Horses-Revised Consensus Statement. J Vet Intern Med. 2016;30:503–515. doi: 10.1111/jvim.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rush BR, et al. Pulmonary function in horses with recurrent airway obstruction after aerosol and parenteral administration of beclomethasone dipropionate and dexamethasone, respectively. Am J Vet Res. 1998;59:1039–1043. [PubMed] [Google Scholar]
- 50.Robinson NE, et al. Relationship between clinical signs and lung function in horses with recurrent airway obstruction (heaves) during a bronchodilator trial. Equine Vet J. 2000;32:393–400. doi: 10.2746/042516400777591147. [DOI] [PubMed] [Google Scholar]
- 51.van Erck E, Votion D, Art T, Lekeux P. Measurement of respiratory function by impulse oscillometry in horses. Equine Vet J. 2004;36:21–28. doi: 10.2746/0425164044864714. [DOI] [PubMed] [Google Scholar]
- 52.Lavoie-Lamoureux A, et al. IL-4 activates equine neutrophils and induces a mixed inflammatory cytokine expression profile with enhanced neutrophil chemotactic mediator release ex vivo. Am J Physiol Lung Cell Mol Physiol. 2010;299:L472–482. doi: 10.1152/ajplung.00135.2009. [DOI] [PubMed] [Google Scholar]
- 53.Dauvillier J, et al. Effect of long-term fluticasone treatment on immune function in horses with heaves. J Vet Intern Med. 2011;25:549–557. doi: 10.1111/j.1939-1676.2011.0717.x. [DOI] [PubMed] [Google Scholar]
- 54.Lavoie-Lamoureux A, Beauchamp G, Quessy S, Martin JG, Lavoie JP. Systemic inflammation and priming of peripheral blood leukocytes persist during clinical remission in horses with heaves. Vet Immunol Immunopathol. 2012;146:35–45. doi: 10.1016/j.vetimm.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs TA, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]


