Abstract
Total RNA and poly(A)+ RNA were isolated from tissues and cultured cells of various mammalian species (bovine muzzle epidermis and bladder urothelium; rat hepatoma cells; human cell lines HeLa, MCF-7 and A-431) and examined by translation in vitro using the reticulocyte lysate system. Polypeptides were separated and identified by two-dimensional electrophoresis and cytokeratins were selectively enriched from the translation assays by co-polymerization with added heterologous cytokeratins. In all three species, non-epidermal cytokeratins A, D and mol. wt. 40,000 (corresponding to numbers 8, 18 and 19 of the human cytokeratin catalog of Moll et al., 1982) were identified as translation products capable of co-polymerization with epidermal keratins. Several other basic and other acidic cytokeratins were also identified as translational products. In addition, two unidentified polypeptides (mol. wt. 52,000 and 43,000) which were minor polypeptides in cytoskeletons and translation assays were found to be specifically enriched in co-polymers with bovine epidermal keratins. The results indicate that many, perhaps all, non-epidermal cytokeratins characteristic of simple epithelia are genuine products of translation and that their diversity is not due to post-translational modification or processing. These findings, taken together with observations of in vitro translation of epidermal mRNAs, suggest that the diversity of cell type-specific expression of the different members of the cytokeratin polypeptide family is largely due to the cell type-specific synthesis of diverse mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. S., Fellini S. A., Croop J. M., Otto J. J., Bryan J., Holtzer H. Differences among 100-A filamentilament subunits from different cell types. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4364–4368. doi: 10.1073/pnas.75.9.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladon P. T., Bowden P. E., Cunliffe W. J., Wood E. J. Prekeratin biosynthesis in human scalp epidermis. Biochem J. 1982 Oct 15;208(1):179–187. doi: 10.1042/bj2080179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Czosnek H., Soifer D., Wisniewski H. M. Studies on the biosynthesis of neurofilament proteins. J Cell Biol. 1980 Jun;85(3):726–734. doi: 10.1083/jcb.85.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P. F., Hong B. S., Cooke P. Classes of distinguishable 10 nm cytoplasmic filaments. Exp Cell Res. 1977 Oct 15;109(2):471–474. doi: 10.1016/0014-4827(77)90033-7. [DOI] [PubMed] [Google Scholar]
- Dodemont H. J., Soriano P., Quax W. J., Ramaekers F., Lenstra J. A., Groenen M. A., Bernardi G., Bloemendal H. The genes coding for the cytoskeletal proteins actin and vimentin in warm-blooded vertebrates. EMBO J. 1982;1(2):167–171. doi: 10.1002/j.1460-2075.1982.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey S. J., Larsen P. M., Bravo R., Celis A., Celis J. E. Differential immunological crossreactivity of HeLa keratin antibodies with human epidermal keratins. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1905–1909. doi: 10.1073/pnas.80.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Vandekerckhove J., Weber K. Permanently proliferating rat vascular smooth muscle cell with maintained expression of smooth muscle characteristics, including actin of the vascular smooth muscle type. J Cell Biol. 1980 Dec;87(3 Pt 1):594–600. doi: 10.1083/jcb.87.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979 Jul;17(3):573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. doi: 10.1073/pnas.78.7.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P. E., Freedberg I. M. Epidermal keratin messenger RNAs: a heterogeneous family. Biochim Biophys Acta. 1982 Feb 26;696(2):124–133. doi: 10.1016/0167-4781(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Gilmartin M. E., Culbertson V. B., Freedberg I. M. Phosphorylation of epidermal keratins. J Invest Dermatol. 1980 Sep;75(3):211–216. doi: 10.1111/1523-1747.ep12522887. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Ma A. Isolation of rat hepatocyte plasma membranes. II. Identification of membrane-associated cytoskeletal proteins. J Cell Biol. 1983 Jan;96(1):230–239. doi: 10.1083/jcb.96.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schmid E., Jorcano J. L., Franke W. W. De novo synthesis and specific assembly of keratin filaments in nonepithelial cells after microinjection of mRNA for epidermal keratin. Cell. 1983 Apr;32(4):1125–1137. doi: 10.1016/0092-8674(83)90296-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Leim R. K., Keith C. H., Leterrier J. F., Trenkner E., Shelanski M. L. Chemistry and biology of neuronal and glial intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):341–350. doi: 10.1101/sqb.1982.046.01.035. [DOI] [PubMed] [Google Scholar]
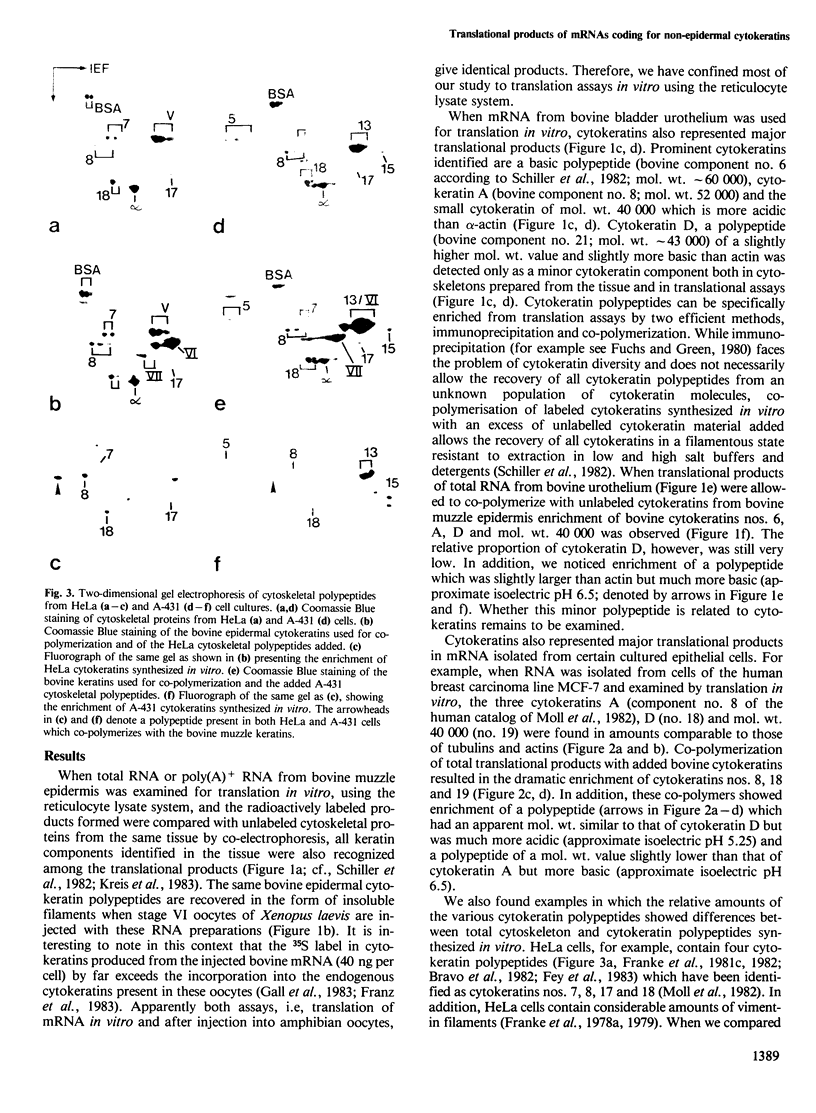
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Asai D. J., Flytzanis C. N., Lazarides E. In vitro translation of the intermediate filament proteins desmin and vimentin. Mol Cell Biol. 1981 Apr;1(4):303–309. doi: 10.1128/mcb.1.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3452–3456. doi: 10.1073/pnas.79.11.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. Heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur J Biochem. 1983 May 16;132(3):477–484. doi: 10.1111/j.1432-1033.1983.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Hawley-Nelson P., Cheng C. K., Yuspa S. H. Keratin gene expression in mouse epidermis and cultured epidermal cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):716–720. doi: 10.1073/pnas.80.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W., Geiger B. A subfamily of relatively large and basic cytokeratin polypeptides as defined by peptide mapping is represented by one or several polypeptides in epithelial cells. EMBO J. 1982;1(6):761–769. doi: 10.1002/j.1460-2075.1982.tb01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W. Limited proteolysis of cytokeratin a by an endogeneous protease: removal of positively charged terminal sequences. Cell Biol Int Rep. 1983 Jan;7(1):3–3. doi: 10.1016/0309-1651(83)90098-x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. N., Pardue R. L., Tutt M. C., Briggs R. C., Brinkley B. R., Hnilica L. S. Identification of cytokeratin antigens in Novikoff ascites hepatoma. Proc Natl Acad Sci U S A. 1982 May;79(10):3138–3142. doi: 10.1073/pnas.79.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Sharp G., Osborn M., Weber K. Occurrence of two different intermediate filament proteins in the same filament in situ within a human glioma cell line. An immunoelectron microscopical study. Exp Cell Res. 1982 Oct;141(2):385–395. doi: 10.1016/0014-4827(82)90227-0. [DOI] [PubMed] [Google Scholar]
- Steinert P., Idler W., Aynardi-Whitman M., Zackroff R., Goldman R. D. Heterogeneity of intermediate filaments assembled in vitro. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):465–474. doi: 10.1101/sqb.1982.046.01.043. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]