Abstract
Nitric oxide (NO) plays an important role in inflammatory joint disease and endothelial function. Endothelial dysfunction has been attributed to a reduction in NO bioactivity in rheumatoid arthritis (RA). However, the relationship of NO with inflammation and endothelial dysfunction in RA has not yet been investigated.
To investigate the relationship of nitrite with inflammation and endothelial dysfunction in RA.
Total 28 patients satisfying 2010 Rheumatoid Arthritis Classification Criteria were recruited for the study. Serum nitrite estimation was performed by Griess reaction. Flow-mediated dilation (FMD) assessed using AngioDefender. Inflammatory disease activity measures included disease activity score of 28 joints (DAS28), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Proinflammatory cytokines (TNF-α, IL-6, and IL-1) measured using standard ELISA kits. Twenty-five healthy controls matched for age and sex were included for comparison.
The serum nitrite level in patients with RA was markedly elevated as compared with controls ( p < 0.05). FMD was significantly impaired in RA patients than controls ( p < 0.05). DAS28 was significantly higher in RA patients ( p < 0.05). Levels of ESR, CRP, TNF-α, IL-1, and IL-6 were significantly higher in RA patients than controls ( p < 0.05). Significant positive correlation was observed between nitrite and CRP ( r = 0.46, p < 0.05), TNF-α ( r = 0.53, p < 0.05), and inverse correlation with FMD ( r =0.62, p < 0.05).
Inflammatory disease activity and endothelial dysfunction in RA are associated with increased concentration of proinflammatory cytokines and NO. Inflammatory triggered release of cytokines induced NO production that mediates endothelial dysfunction. These findings suggest a role for NO in inflammation-induced endothelial dysfunction in RA.
Keywords: endothelial dysfunction, inflammation, nitric oxide, proinflammatory cytokines, rheumatoid arthritis
Rheumatoid arthritis (RA) patients have an increased risk of atherosclerotic cardiovascular disease that cannot be explained by traditional cardiovascular risk factors alone. 1 2 Because of some similarities between inflammation/autoimmune diseases and atherosclerosis, it has been suggested that inflammatory mediators may contribute to vascular dysfunction in patients with RA. 3 The healthy vascular endothelium has vasodilator, antiadhesive, anti-inflammatory, and anticoagulant properties, through the production of mediators, including nitric oxide (NO). Endothelial dysfunction (ED) is an early event in the pathogenesis of atherosclerosis 4 5 and characterized by reduced dilator function, increased inflammatory cell and platelet adhesion, 6 and increased coagulation activity. 7 Reduced bioavailability of NO makes a major contribution to ED, and it may be caused by decreased expression of the endothelial cell NO synthase (eNOS), a lack of substrate or cofactors for eNOS, alterations of cellular signaling such that eNOS is not appropriately activated, and accelerated NO degradation by reactive oxygen species (ROS). 8 9 10 11 One potential trigger for ED is inflammation. Inflammatory cytokines impair endothelial function in animal models 12 and isolated human veins. 13
NO is an important mediator of immunity and inflammation that is responsible for the inhibition of platelet adhesion and endothelial vasorelaxation. 14 It is generated from l -arginine by three different nitric oxide synthase (NOS) isoforms in many cells. Type I neuronal (nNOS), type II inducible (iNOS), and type III endothelial (eNOS). Only one of them (iNOS) is a Ca 2+ -independent isoform; it can be induced by cytokines during inflammatory processes and generates large amounts of NO. Since NO is also synthesized by eNOS in the endothelium and ED occurs in RA. 15 However, the relationship of NO with inflammation and ED in RA has not yet been investigated. It is important to investigate the relationship between inflammation and ED with respect to NO in RA.
Methods
Patients
This was a cross-sectional study of 28 patients with RA (5 males, 23 females), recruited from the rheumatology outpatient clinic. All patients satisfied the 2010 Rheumatoid Arthritis Classification Criteria for diagnosis and classification of RA 16 with active disease despite treatment with conventional disease-modifying antirheumatic drugs (DMARDs). All the patients included had active RA, defined by the presence of modified disease activity score of 28 joints (DAS28 >3.2).
Exclusion criteria included patients with diabetes mellitus, hepatic and renal failure, peripheral artery disease, stroke, coronary artery disease, thyroid disorders, hypertension, and smokers. None of the patients were receiving nitrates, statins, β-blockers, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, or tumor necrosis factor-α (TNF-α) inhibitors that have been shown to affect the ED. Twenty-five healthy patients matched for age (mean age: 43.8 ± 5.3) and sex (4 males, 21 females) acted as control.
The study protocol was approved by the regional ethical research committee and was performed in accordance with the declaration of Helsinki and the code of Good Clinical Practice. All patients provided written informed consent to participate after a full explanation of the study.
Assessment
In all patients, the following hematologic and other laboratory tests were determined by established protocol using standard kits: complete blood count, liver and renal function tests, thyroid-stimulating hormone, and fasting blood sugar.
Serum Nitrite Concentration
NO was directly determined as nitrite by a spectrophotometric method, using serum samples collected after at least 14 hours of fasting. 17 Gaseous NO-free radical is rapidly metabolized to nitrate or nitrite; hence measured nitrite concentration is taken as an index of NO production. 17 Nitrite is also not influenced by dietary variation. 18 All the patients were teetotallers and nonsmokers and taking a normal regular diet. Patients were asked to fast after the evening meal at 6.00 pm till 8.00 am next morning, when the sample was obtained. Samples were estimated in duplicate and mean of two estimations was considered for further assessments.
Assessment of Inflammatory Disease Activity
DAS28 was used to assess disease activity of 28 joints.
Erythrocyte sedimentation rate (ESR) was measured by Westergren method and C-reactive protein (CRP) level was determined using standard commercial kits.
Estimation of proinflammatory cytokines, that is, TNF-α, interleukin-6 (IL-6), and interleukin-1 (IL-1), was done using standard enzyme-linked immunosorbent assay (ELISA) kits (Diaclone SAS, France).
Assessment of Endothelial Function by AngioDefender
The AngioDefender (Everist Genomics, Ann Arbor, Michigan) procedure is noninvasive and uses neither ultrasound nor Doppler flow analysis. The AngioDefender device uses a novel, proprietary software algorithm to analyze pulse wave data collected before and after brachial artery occlusion by an upper arm sphygmomanometric cuff. At the end of the AngioDefender testing procedure (∼15 minutes), the maximal relative postocclusion change in the diameter of the brachial artery relative to baseline is calculated and expressed as a percentage of flow-mediated dilation (%FMD). 19 AngioDefender testing is applicable to any patient, regardless of age, sex, ethnicity, or preexisting conditions. AngioDefender test results are not dependent on user technique or operator proficiency.
Statistical Analysis
Test values are reported as mean ± SD (standard deviation). Spearman analysis was used to find the relationship between nitrite, inflammatory disease activity measures, proinflammatory cytokines, and FMD. A p < 0.05 was considered to indicate significant difference. Statistical analysis was done using the Sigmastat 3.5 (Systat Software, San Jose, CA) for Windows 7.0.
Results
Patient Profile
The baseline demographic and clinical characteristics of the patients and controls are presented in Table 1 .
Table 1. The demographic and clinical characteristics of the RA patients and healthy controls.
| Patient characteristics | RA ( n = 28) |
Control ( n = 25) |
p Value |
|---|---|---|---|
| Sex (F:M) | 23:5 | 21:4 | |
| Age (y) | 44.03 ± 6.9 | 43.8 ± 5.3 | 0.34 |
| Disease duration (y) | 7.17 ± 5.32 | ||
| BMI (kg/m 2 ) | 22.67 ± 2.80 | 21.73 ± 2.91 | 0.37 |
| ESR (mm 1st h) | 38.57 ± 7.09 | 18.55 ± 2.43 | 0.01 a |
| CRP (mg/dL) | 13.57 ± 5.30 | 5.51 ± 2.12 | 0.03 a |
| DAS 28 Score | 4.24 ± 0.69 | ||
| TNF-α (pg/mL) | 5.91 ± 1.14 | 2.86 ± 0.12 | 0.01 a |
| IL-6 (pg/mL) | 16.46 ± 3.34 | 9.79 ± 2.22 | 0.01 a |
| IL-1 (pg/mL) | 206.5 ± 24.7 | 143.5 ± 14.09 | 0.01 a |
| FMD (%) | 4.90 ± 1.27 | 9.7 ± 2.1 | 0.01 a |
| Nitrite (µmol/L) | 6.01 ± 1.12 | 3.84 ± 0.82 | 0.01 a |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; DAS28, disease activity score of 28 joints; ESR, erythrocyte sedimentation rate; F, female; FMD, flow-mediated vasodilation; IL-1, interleukin-1; IL-6, interleukin-6; M, male; RA, rheumatoid arthritis; TNF-α, tumor necrosis factor-α.
p < 0.05, statistically significant.
Serum Nitrite Concentration
Serum nitrite levels were higher in RA patients (6.01 ± 1.1) when compared with healthy controls (3.84 ± 0.22) ( p < 0.01) at baseline ( Table 1 ).
Inflammatory Disease Activity
All patients included in the study had active RA (DAS28 ≥ 3.2). Levels of inflammatory disease activity measures, that is, DAS28 ( p = 0.01), ESR ( p = 0.01), and CRP ( p = 0.03), were significantly higher in RA patients as compared with healthy controls ( Table 1 ).
Levels of proinflammatory cytokines, that is, TNF-α ( p = 0.01), IL-6 ( p = 0.02), and IL-1 ( p = 0.04) were significantly higher in RA patients as compared with controls ( Table 1 ).
Endothelial Function
FMD in RA patients was significantly impaired as compared with the healthy age and sex matched control group (4.90 ± 1.27% vs. 9.7 ± 2.1%, p < 0.005) ( Table 1 ).
Association of Serum Nitrite with Disease Activity Measures, Proinflammatory Cytokines and Endothelial Dysfunction
At univariate analysis, serum nitrite was positively correlated with CRP ( p = 0.01) and TNF-α ( p = 0.03) ( Fig. 1 ), and inversely correlated with FMD ( p = 0.01) ( Fig. 2 ) in RA ( Table 2 ). The correlation between the nitrite increase and the degree of vasodilation suggests that nitrite changes may reflect endothelial function.
Fig. 1.
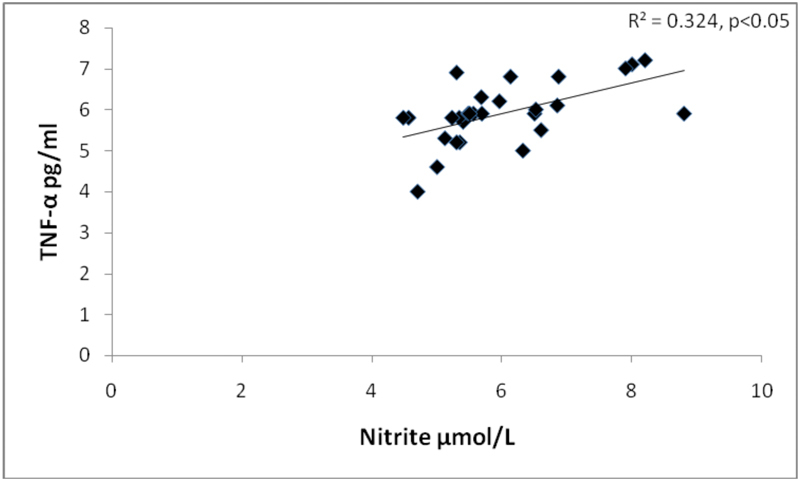
Correlation of nitrite with tumor necrosis factor-α (TNF-α) in RA patients.
Fig. 2.
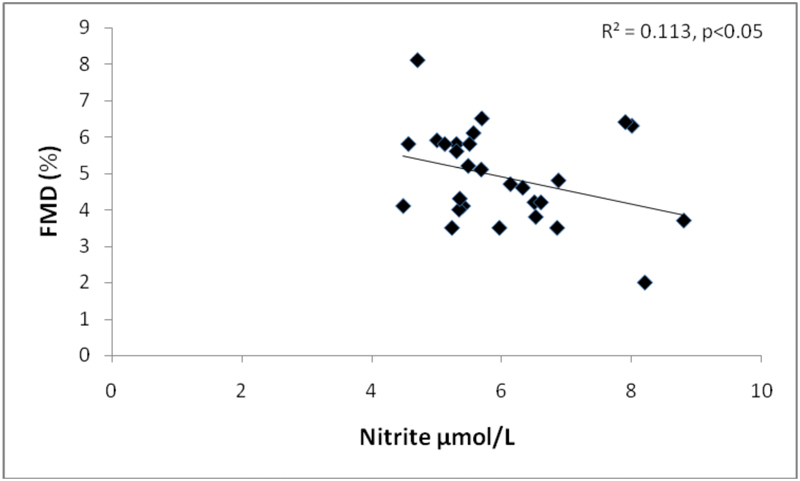
Correlation of nitrite with flow-mediated dilation (FMD) in RA patients.
Table 2. Univariate analysis of serum nitrite with various selected variables.
| Variables | r | p Value |
|---|---|---|
| Age | 0.23 | 0.22 |
| Disease duration | 0.26 | 0.34 |
| DAS 28 | 0.39 | 0.08 |
| ESR | 0.11 | 0.55 |
| CRP | 0.46 | 0.01 a |
| IL-1 | 0.32 | 0.09̀ |
| IL-6 | 0.35 | 0.07 |
| TNF-α | 0.53 | 0.03 a |
| FMD | −0.62 | 0.001 a |
Abbreviations: CRP, C-reactive protein; DAS28, disease activity score of 28 joints; ESR, erythrocyte sedimentation rate; FMD, flow-mediated dilation; IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
p < 0.05, statistically significant.
Discussion
RA is an autoimmune and chronic inflammatory condition, associated with ED and increased risk of cardiovascular disease. 1 In addition, patients with RA have a dual abnormality in NO-dependent vascular function, characterized by blunted endothelial NO synthase (eNOS) and enhanced inducible NO synthase (iNOS) by leukocytes and vascular smooth muscle cells (VSMCs). 20 Induction of iNOS of the infiltrated cells, synovial lining layer, cartilage, chondrocytes, and fibroblasts by proinflammatory cytokines (IL-1, IL-6, TNF-α, and TGF-b) release large amounts of NO, which leads to local (synovial fluid) and systemic increases in nitrite and nitrate in systemic autoimmune disease. 21 22 The present study demonstrates the association of the increased serum nitrite (NO surrogate) in patients suffering from active RA with disease activity, inflammatory cytokines, and endothelial function. This, as far as we know, is the first time that such a relationship has been investigated in RA.
In the present study, elevated serum nitrite concentrations were noticed in RA patients compared with the controls. This adds to the in vitro 23 and in vivo reports 24 that demonstrate increase in nitrite levels of the synovial fluid, urine, or serum of the patients with active RA. This increase in nitrite may be attributed to widespread synovial inflammation in patients with RA. In RA, cells such as fibroblast, cartilage, chondrocytes, and synovial lining layer, triggered by local cytokine release, contribute part of elevated NO. 21 22 The circulatory cytokines also increase NO synthesis by inducing the expression of iNOS of endothelial cells, infiltrated cells, and immune cells in RA. 21 iNOS activity is very low in normal conditions and stimulated during inflammation, and the amount of NO produced by iNOS may be 1,000-fold greater than that produced by endothelial NOS, leading to local (synovial fluid) and systemic increase in nitrite and nitrate concentrations. 25
In the present study, higher concentration of proinflammatory cytokines (TNF-α, IL-1, IL-6) were found in RA patients compared with controls, which is thought to be responsible for increase nitrite level in RA. Our previous studies have also shown elevated levels of proinflammatory cytokines (TNF-α, IL-1, IL-6) in RA. 26 27 In patients with active RA, blood mononuclear cells had increased NO synthase activity due to expression of the iNOS after stimulation by proinflammatory cytokines. 28 Only iNOS is a Ca 2+ -independent isoform; it can be induced by cytokines during inflammation and generates large amounts of NO. 29 A positive correlation of nitrite with TNF-α in our study suggests that this cytokine may be involved in the upregulation of T cells and endothelial cells that increase the expression of iNOS and are responsible for increased production of NO in RA. T cells from RA patients produce more than 2.5 times more NO than healthy donor T cells. 30 Another possible mechanism is also there that in RA, nuclear factor-kappa B (NF-κB) is overexpressed in the inflamed synovium, 31 where its activity may enhance recruitment of inflammatory cells and production of proinflammatory mediators such as IL-1, IL-6, IL-8, and TNF-α that is responsible for increase expression of iNOS.
In the present study, increased disease activity (DAS28) and higher levels of ESR and CRP in RA as compared with controls depicts enhanced inflammatory disease activity responsible for increased nitrite levels in RA. Positive correlations of serum nitrite with CRP ( r = 0.69, p < 0.001) showed that patients with higher CRP levels had higher nitrite concentration. These results are in accordance with a previous study, which has shown an association between CRP and nitrite in RA. 32 This result may provide indirect evidence of induction of iNOS as well as hepatic synthesis and release of CRP by proinflammatory cytokines. Furthermore, it may show that these cytokines are also elevated in the circulating system that may induce the production and release of CRP by hepatocytes. It has been demonstrated in in vitro studies that CRP is also capable of stimulating the production of NO, independent of iNOS stimulation. 33 This finding suggests the existence of an inflammatory substrate in the etiopathogenesis of RA and CRP (as it is a biomarker of inflammation in RA) is responsible for the loss of balance in the endothelial NO system and the subsequent ED.
A reduction in the FMD, reflecting ED, has been demonstrated in early stages of atherosclerosis and in patients with inflammatory rheumatic diseases such as ankylosing spondylitis (AS) and RA. 19 24 In this study, we corroborated these results, finding a reduction in FMD in patients with RA with respect to controls. Increased production of NO in RA due to the activation of iNOS and an attenuation of eNOS activity reduces basal FMD. The inverse correlation between nitrite and FMD found in this study implies a relationship between nitrite, inflammation, and ED in the etiopathogenesis of RA, with the loss of the homeostatic function of NO as a key step in the origins of the disease, but not in its progression. ED resulting in reduced level of NO activity can lead not only to an imbalance in vascular tone, favoring acute vasoconstriction, but can also impair an endogenous negative feedback loop that limits vascular cell adhesion molecule-1 (VCAM-1) expression, leukocyte adhesion, and atherogenesis. 34 35 Our study provides a link between NO production and ED, and offers clues as to the mechanism of such an effect.
Conclusion
In conclusion, this is the first study to show the relationship between inflammation and ED with respect to NO. Inflammatory triggered release of cytokines induced NO production that leads to ED. We can therefore conclude that inflammation-triggered release of proinflammatory cytokines (IL-1, IL-6, TNF-α) in RA induced the production of NO that mediates ED possibly through upregulation of adhesion molecules. Finally, our results indicate that the measurement of nitrite could be a diagnostic, as well as prognostic, tool during the treatment of RA patients. This would also serve as a novel therapeutic target for preventing cardiovascular risk associated with impaired ED in RA.
Footnotes
Conflicts of Interest The authors have none to declare.
References
- 1.Wolfe F, Mitchell D M, Sibley J T et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(04):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.Myllykangas-Luosujärvi R, Aho K, Kautiainen H, Isomäki H. Cardiovascular mortality in women with rheumatoid arthritis. J Rheumatol. 1995;22(06):1065–1067. [PubMed] [Google Scholar]
- 3.Manzi S, Wasko M C. Inflammation-mediated rheumatic diseases and atherosclerosis. Ann Rheum Dis. 2000;59(05):321–325. doi: 10.1136/ard.59.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creager M A, Cooke J P, Mendelsohn M E et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86(01):228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg H O, Bayazeed B, Hook G, Johnson A, Cronin J, Baron A D. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation. 1997;96(10):3287–3293. doi: 10.1161/01.cir.96.10.3287. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith I R, Blann A D, Patel R L, Lip G Y. Plasma fibrinogen, soluble P-selectin, and von Willebrand factor in aortic valve disease: evidence for abnormal haemorheology, platelet activation, and endothelial dysfunction. Heart. 2000;83(05):577–578. doi: 10.1136/heart.83.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bombeli T, Mueller M, Haeberli A. Anticoagulant properties of the vascular endothelium. Thromb Haemost. 1997;77(03):408–423. [PubMed] [Google Scholar]
- 8.Wilcox J N, Subramanian R R, Sundell C L et al. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17(11):2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- 9.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267(34):24173–24176. [PubMed] [Google Scholar]
- 10.Shimokawa H, Flavahan N A, Vanhoutte P M. Loss of endothelial pertussis toxin-sensitive G protein function in atherosclerotic porcine coronary arteries. Circulation. 1991;83(02):652–660. doi: 10.1161/01.cir.83.2.652. [DOI] [PubMed] [Google Scholar]
- 11.Harrison D G.Endothelial function and oxidant stress Clin Cardiol 199720(11, Suppl 2)II-11–II-17. [PubMed] [Google Scholar]
- 12.Ohkawa F, Ikeda U, Kanbe T, Kawasaki K, Shimada K. Effects of inflammatory cytokines on vascular tone. Cardiovasc Res. 1995;30(05):711–715. [PubMed] [Google Scholar]
- 13.Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96(09):3042–3047. doi: 10.1161/01.cir.96.9.3042. [DOI] [PubMed] [Google Scholar]
- 14.Buldanlioglu S, Turkmen S, Ayabakan H B et al. Nitric oxide, lipid peroxidation and antioxidant defence system in patients with active or inactive Behçet's disease. Br J Dermatol. 2005;153(03):526–530. doi: 10.1111/j.1365-2133.2005.06543.x. [DOI] [PubMed] [Google Scholar]
- 15.Totoson P, Maguin-Gaté K, Prati C, Wendling D, Demougeot C. Mechanisms of endothelial dysfunction in rheumatoid arthritis: lessons from animal studies. Arthritis Res Ther. 2014;16(01):202. doi: 10.1186/ar4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aletaha D, Neogi T, Silman A J et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(09):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 17.Sastry K VH, Moudgal R P, Mohan J, Tyagi J S, Rao G S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306(01):79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 18.Knight T M, Forman D, Al-Dabbagh S A, Doll R. Estimation of dietary intake of nitrate and nitrite in Great Britain. Food Chem Toxicol. 1987;25(04):277–285. doi: 10.1016/0278-6915(87)90123-2. [DOI] [PubMed] [Google Scholar]
- 19.Garg N, Krishan P, Syngle A. Rosuvastatin improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol. 2015;34(06):1065–1071. doi: 10.1007/s10067-015-2912-3. [DOI] [PubMed] [Google Scholar]
- 20.van Leuven S I, Franssen R, Kastelein J J, Levi M, Stroes E S, Tak P P. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47(01):3–7. doi: 10.1093/rheumatology/kem202. [DOI] [PubMed] [Google Scholar]
- 21.Ralston S H. The Michael Mason Prize Essay 1997. Nitric oxide and bone: what a gas! Br J Rheumatol. 1997;36(08):831–838. doi: 10.1093/rheumatology/36.8.831. [DOI] [PubMed] [Google Scholar]
- 22.Bertero M T, Caligaris-Cappio F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica. 1997;82(03):375–381. [PubMed] [Google Scholar]
- 23.Wigand R, Meyer J, Busse R, Hecker M. Increased serum NG-hydroxy-L-arginine in patients with rheumatoid arthritis and systemic lupus erythematosus as an index of an increased nitric oxide synthase activity. Ann Rheum Dis. 1997;56(05):330–332. doi: 10.1136/ard.56.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ersoy Y, Ozerol E, Baysal O et al. Serum nitrate and nitrite levels in patients with rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. Ann Rheum Dis. 2002;61(01):76–78. doi: 10.1136/ard.61.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syngle A, Vohra K, Kaur L, Sharma S. Effect of spironolactone on endothelial dysfunction in rheumatoid arthritis. Scand J Rheumatol. 2009;38(01):15–22. doi: 10.1080/03009740802279709. [DOI] [PubMed] [Google Scholar]
- 26.Garg N, Syngle A, Verma I, Krishan P. Endothelial progenitor cells as cardiovascular surrogate markers in seropositive rheumatoid arthritis. IJRCI. 2015;3(01):OA1. [Google Scholar]
- 27.Syngle A, Garg N, Krishan P. Rosuvastatin improves endothelial progenitor cells in rheumatoid arthritis. IJAR. 2014;2:959–966. [Google Scholar]
- 28.St Clair E W, Wilkinson W E, Lang T et al. Increased expression of blood mononuclear cell nitric oxide synthase type 2 in rheumatoid arthritis patients. J Exp Med. 1996;184(03):1173–1178. doi: 10.1084/jem.184.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundberg J O, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25(05):915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 30.Nagy G, Clark J M, Buzas E et al. Nitric oxide production of T lymphocytes is increased in rheumatoid arthritis. Immunol Lett. 2008;118(01):55–58. doi: 10.1016/j.imlet.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Bingham C O., III The pathogenesis of rheumatoid arthritis: pivotal cytokines involved in bone degradation and inflammation. J Rheumatol Suppl. 2002;65:3–9. [PubMed] [Google Scholar]
- 32.Kaur J, Syngle A, Krishan P et al. IL-6 inhibition improves nitric oxide in rheumatoid arthritis. IJRCI. 2014;2(01):OA2. [Google Scholar]
- 33.Clapp B R, Hirschfield G M, Storry C et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111(12):1530–1536. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]
- 34.Ignarro L J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 35.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(02):109–142. [PubMed] [Google Scholar]


