Abstract
The involvement of oxidative stress in protocatechuic acid‐mediated bacterial lethality was investigated. Minimum inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC) of protocatechuic acid against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus are 600 and 700 μg/ml, 600 and 800 μg/ml, and 600 and 800 μg/ml, respectively. The optical densities and colony‐forming units of protocatechuic acid‐treated bacteria decreased in time‐dependent manner. Protocatechuic acid (4× MIC) significantly increased the superoxide anion content of E. coli, P. aeruginosa, and S. aureus compared to dimethyl sulfoxide (DMSO). Superoxide dismutase, catalase, and NAD+/NADH in protocatechuic acid‐treated E. coli, P. aeruginosa, and S. aureus increased significantly when compared to DMSO. Conversely, level of reduced glutathione decreased in protocatechuic acid‐treated E. coli, P. aeruginosa, and S. aureus, while glutathione disulfide increased when compared to DMSO. Furthermore, malondialdehyde and fragmented DNA increased significantly following exposure to protocatechuic acid. Protocatechuic acid inhibited the activity of complexes I and II. From the above findings, protocatechuic acid enhanced the generation of reactive oxygen species (superoxide anion radical and hydroxyl radical) in E. coli, P. aeruginosa, and S. aureus, possibly by autoxidation, fenton chemistry, and inhibiting electron transport chain resulting in lipid peroxidation and DNA fragmentation and consequentially bacterial cell death.
Keywords: Antioxidant enzymes; bacteria; electron transport chain complex; fragmented DNA, hydroxyl radical, NAD+/NADH; protocatechuic acid, superoxide anion radical
1. Introduction
Reactive oxygen species, such as superoxide anion radical, hydrogen peroxide, and hydroxyl radical, are inevitable by‐products of aerobic respiration (Storz & Imlay, 1999). These oxygen‐derived species possess potent antimicrobial activity (Dixon & Stockwell, 2014; Fang, 2011), which largely results from perturbation of antioxidant and prooxidant balance in favor of latter. Redox perturbation in bacteria occurs from both exogenously and endogenously generated ROS that overwhelm bacterial antioxidant defense system. While environmental chemicals with prooxidant potentials form exogenous source of ROS, mitochondrial electron transport chain is the chief source of endogenously generated ROS (Nickel, Kohlhaas, & Maack, 2014). The primary targets of these ROS are cellular protein, lipids, and DNA.
Recent studies have documented the involvement of ROS in bacterial lethality of antibiotics and antimicrobials (Ajiboye et al., 2016a,b,c,d; Kohanski, Dwyer, Hayete, Lawrence, & Collins, 2007). In these studies, hydroxyl radical, generated through the catalytic action of ferrous ion on hydrogen peroxide (Fenton chemistry) (Lemire, Harrison, & Turner, 2013), is responsible for bacterial death. Plant derived phytochemicals, including phenolics with catechol ring, possess prooxidant activity (Schweigert, Zehnder, & Eggen, 2001). However, no studies have reported any relationship between their capability to generate ROS and bacterial lethality.
Protocatechuic acid (3,4‐dihydroxybenzoic acid) is a benzoic acid derivative found in vegetables, nuts, brown rice, fruits, and herbal medicines (Da‐Costa‐Rocha, Bonnlaender, Sievers, Pischel, & Heinrich, 2014). Studies have demonstrated the anticarcinogenic, antioxidants, cytotoxic, free radical scavenging, apoptotic, and cell cycle arrest activities (Ferreira, Barros, & Abreu, 2009; Yin, Lin, Wu, Tsao, & Hsu, 2009; Yip, Chan, Pang, Tam, & Wong, 2006). Experimental findings have also demonstrated the prooxidant activity of protocatechuic acid in cell‐free and in vitro cellular systems (Simić, Manojlović, Šegan, & Todorović, 2007; Zeraik et al., 2014). In this study, we demonstrated that protocatechuic acid promotes redox‐related biochemical changes leading to death of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus.
2. Materials and Methods
2.1. Bacteria strains
Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 29213) were procured from American Type Culture Collection and propagated on a Luria–Bertani (LB) at 37°C.
2.2. Chemicals
Dimethyl sulfoxide, diphenylamine, 5,5′‐dithiobis(2‐nitrobenzoic acid), epinephrine, guanidine hydrochloride, hydrogen peroxide, N‐ethylmaleimide, sodium chloride, thiobarbituric acid were procured from Sigma‐Aldrich (St. Louis, MO). Nitroblue tetrazolium, 2,2′‐dipyridyl, l‐glutathione (reduced), and protocatechuic acid are products of Santa Cruz Biotechnology. All other reagents are products of Sigma‐Aldrich (St. Louis, MO).
2.3. Minimum inhibitory concentration and minimum bactericidal concentration
Minimum inhibitory concentration (MIC) of protocatechuic acid was determined using 96‐well microtiter plate as described by Balouiri, Sadiki, and Ibnsouda (2016). Inocula (104 CFU/ml) were mixed with protocatechuic acid in 96‐well microtiter plate to give concentrations (1–109 ng/ml). The culture medium containing 0.04% DMSO in sterile distilled water served as control. Plates were incubated for 24 hr at 37°C. MIC was expressed as the lowest concentration which inhibited growth, judged by lack of turbidity in the well. The turbidity in each well was then determined using Versamax microplate reader (Molecular Devices, CA, USA). The experiment was performed in triplicate and repeated three times along with reference antibiotics, ciprofloxacin. The content of wells lacking turbidity were aspirated into eppendorf tubes, and centrifuged to collect the cells. The cells were washed to aseptically remove protocatechuic acid, diluted with 0.9% NaCl, mixed with molten soft LB agar (0.8%) at 42°C, and poured onto agar plates containing solid LB agar (1.5%). Colonies were counted after 24 hr at 37°C. The concentration that completely inhibited colony formation, despite the treatment with test compound for 48 hr, was aseptically removed and transferred into a new agar plate to examine survival at 24 hr.
2.4. Time–kill bacterial susceptibility assay
Susceptibility of E. coli, P. aeruginosa, and S. aureus to protocatechuic acid was investigated using the procedure described by Ajiboye et al. (2016b). Briefly, organisms were grown overnight in LB medium, harvested by centrifugation, and resuspended in 50 ml fresh medium (LB) to OD600 = 0.1, and grown aerobically at 37°C in 250 ml flask to OD600 = 0.2. Protocatechuic acid was added to the culture to obtain concentration (4× MIC) or dimethyl sulfoxide (DMSO) and incubated at 37°C for 3 hr. Absorbance of the incubation medium was read at 600 nm for every 30 min interval of 3 hr incubation time. For colony formation, samples of control culture (DMSO‐treated culture) and cultures treated with protocatechuic acid (4× MIC) were removed at intervals (0, 30, 60, 90, 120, 150, and 180 min) and centrifuged to collect the cells as pellet. The cells were washed and diluted with 0.9% NaCl, mixed with molten soft LB agar (0.8%) at 42°C, and poured onto agar plates containing solid LB agar (1.5%). Colonies were counted after 24 hr at 37°C.
2.5. Preparation of cell‐free extract
Cell‐free extract was prepared from the samples obtained after 3 hr incubation of organisms with protocatechuic acid (4× MIC). Cells were harvested by centrifugation, washed twice, and suspended in sucrose‐Tris buffer (25 mmol/L sucrose solution, 10 mmol/L Tris‐HCl, pH 7.4). Glass beads (2 g) were added to the bacterial suspension, homogenized, and centrifuged at 3,000 g for 10 min at 4°C to obtain the cell‐free extract as the supernatant.
2.6. Oxidative stress biomarkers
2.6.1. Superoxide dismutase
The activity of superoxide dismutase (SOD) was determined according to Misra and Fridovich (1972). Briefly, 10 μl of cell‐free extract was added to 125 μl of 0.05 mol/L carbonate buffer (pH 10.2) to equilibrate, and the reaction was started by addition of 15 μl of freshly prepared 0.3 mmol/L epinephrine. The increase in absorbance at 480 nm was recorded every 30 s for 150 s using Versamax microplate reader (Molecular Devices). One unit of enzyme activity was defined as 50% inhibition of the rate of autoxidation of epinephrine as determined by change in absorbance min−1 at 480 nm.
2.6.2. Catalase
The cell‐free extract was evaluated for catalase activity using the procedure described by Chen, Liu, Zhu, Xu, and Li (2010). Briefly, cell‐free extract (10 μl) was mixed thoroughly with cold 6 mmol/L H2O2 (100 μl). The reaction was stopped by mixing with 3 mol/L H2SO4 (20 μl) followed by 0.01 KMnO4. Reaction mixture was vortexed and absorbance read at 480 nm within 30–60 s using Versamax microplate reader (Molecular Devices).
2.6.3. Reduced glutathione (GSH) and glutathione disulfide (GSSG)
The level of GSH in the cell‐free extract was determined using the procedure described by Ellman (1959). Cell‐free extract (20 μl) was mixed with 170 μl of 0.1 mol/L potassium phosphate buffer (pH 7.4). The reaction was started by adding 10 mmol/L DTNB (10 μl) and incubated for 30 min at room temperature. Absorbance of the reaction mixtures was read at 412 nm using Versamax microplate reader (Molecular Devices).
GSSG level was determined using the procedure described by Hissin and Hilf (1976). Cell‐free extract (50 μl) was mixed with 20 μl of 0.04 mol/L N‐ethylmaleimide (NEM) to prevent oxidation of GSH to GSSG. It was incubated at room temperature for 30 min and 1.68 ml of 0.3 mol/L Na2HPO4 solution was added to it followed by 250 μl of DTNB reagent. The absorbance of the sample was measured at 412 nm.
2.6.4. NAD+/NADH
The NAD+/NADH ratio of bacteria cells was assessed using the Sigma‐Aldrich assay kit (MAK037). Cells were washed with cold phosphate‐buffered saline and centrifuged at 2,000g for 5 min. Cell was extracted with 400 μl of NAD+/NADH extraction buffer by homogenization or freeze/thawing for two cycles of 20 min on dry ice followed by 10 min at room temperature. To remove insoluble material, the samples were vortexed for 10 sec and then centrifuged at 13,000g for 10 min. Extracted NAD+/NADH supernatant was transferred into a labeled tube. The supernatant was then used for NAD+/NADH assay.
2.6.5. Malondialdehyde
Malondialdehyde content of cell‐free extract was determined as described by Reilly and Aust (2001). Briefly, cell‐free extract was mixed with TBA/TCA/HCl (15%, 0.37%, 0.2 N) at a reagent/sample ratio of 2:1 (v/v), placed in a boiling water bath for 15 min, cooled to room temperature, and centrifuged at 1,000 g for 10 min at room temperature. The absorbance of the solution was read at 535 nm against the blank (containing all reagents except hepatocytes suspension) using Versamax microplate reader (Molecular Devices). MDA content was determined using the extinction coefficient of 1.56 × 106.
2.6.6. Fragmented DNA
The percentage fragmented DNA in the cell‐free extract was determined using the procedure described by Burton (1956). Briefly, cell‐free extract was centrifuged at 15,000g for 15 min at 4°C. The supernatant was separated from the pellet and treated with trichloroacetic acid (1.50 ml, 10%). The pellet was also treated with trichloroacetic acid (0.65 ml, 5%). The reaction mixtures were allowed to precipitate overnight (≥4 hr) in a refrigerator (4°C), and centrifuged at 2,500 g for 10 min. The reaction mixtures were boiled at 100°C for 15 min, cooled to room temperature, and centrifuged at 2,500 g for 5 min. The supernatants (50 μl) were treated with diphenylamine reagent (100 μl) and incubated at 37°C for 4 hr. Absorbance was read at 600 nm using Versamax microplate reader (Molecular Devices). The fragmented DNA was calculated using the following expression:
2.6.7. Electron transport chain complexes
The activities of NADH:ubiquinone oxidoreductase (complex I; EC 1.6.5.3) and succinate:ubiquinone oxidoreductase (complex II; EC 1.3.5.1) in the cell‐free extract of E. coli, P. aeruginosa, and S. aureus were assayed using the procedures described by Van Bergen, Blake, Crowston, and Trounce (2014). Activities of NADH:ubiquinone oxidoreductase and succinate:ubiquinone oxidoreductase were calculated using extinction coefficients 6.22 and 19.1 mmol/L−1 cm−1, respectively.
2.7. Involvement of reactive oxygen species (superoxide anion radical and hydroxyl radical) in bacterial lethality
2.7.1. Superoxide anion radical
For superoxide anion radical generation, cells (1 ml) in exponential phase were incubated with protocatechuic acid for 30 min, followed by adding nitroblue tetrazolium (0.5 ml, 1 mg/ml) and incubated for 30 min at 37°C. After incubation, 0.1 ml of HCl (0.1 mol/L) was added and centrifuged at 1,500g for 10 min. The reduced nibtroblue tetrazolium in the pellets was extracted with DMSO, diluted with 0.8 ml phosphate‐buffered saline (pH 7.5) and the absorbance was read at 575 nm using Versamax microplate reader (Molecular Devices). The superoxide anion of the cells was calculated using the molar extinction coefficient of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide formazan (17,000 M−1 cm−1 at pH 7.4–8.0) as described by Ajiboye et al. (2016b).
2.7.2. Hydroxyl radical
In this assay, E. coli, P. aeruginosa, and S. aureus were grown overnight in LB medium, harvested by centrifugation and resuspended in 50 ml fresh medium (LB) to OD600 = 0.1, and grown aerobically at 37°C in 250 ml flask. At mid‐log phase (OD600 = 0.5), 2,2′‐dipyridyl (500 μmol/L), thiourea (150 mmol/L), and/or protocatechuic acid (4× MIC) were added and incubated at 37°C for 3 hr. Absorbance of the incubation medium was read at 600 nm for every 20 min interval of 3 hr incubation time using Versamax microplate reader (Molecular Devices). In addition, samples of control culture and treated culture were removed at intervals of 0, 20, 40, 60, 80, 100, 120, 140, 160, and 180 min and centrifuged to collect the cells as pellet. The cells were washed and diluted with 0.9% NaCl, mixed with molten soft LB agar (0.8%) at 42°C, and poured onto agar plates containing solid LB agar (1.5%). Colonies were counted using digital colony counter after 24 hr at 37°C.
2.8. Statistical analysis
Results were expressed as the mean of three independent experiments ± standard deviation. One‐way analysis of variance (ANOVA) followed by Student's t‐test was used to detect any significant difference (p < .05) between the treatments using Statplus, 2011 (AnalystSoft, Inc., Alexandria, VA, USA).
3. Results
3.1. Minimum inhibitory concentration and minimum bactericidal concentration
MIC and MBC are widely used in determining the potency of antimicrobial agents. We determined the MIC and MBC values of protocatechuic acid against E. coli, P. aeruginosa, and S. aureus (Table 1). The MIC and MBC values of protocatechuic acid are relatively higher than that of synthetic reference antibiotic, ciprofloxacin. This is not surprising as a lower MIC and MBC could pose threat to human, since this compound is widely available in fruits at higher concentrations.
Table 1.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of protocatechuic acid
| Protocatechuic acid (μg/ml) | Ciprofloxacin (μg/ml) | |||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Escherichia coli ATCC 25922 | 550 | 600 | 18 | 64 |
| Pseudomonas aeruginosa ATCC 27853 | 300 | 400 | 36 | 64 |
| Staphylococcus aureus ATCC 29213 | 450 | 500 | 36 | 64 |
3.2. Time–kill bacterial susceptibility
The change in absorbance of protocatechuic acid (4× MIC)‐treated E. coli, P. aeruginosa, and S. aureus decreased significantly in time‐dependent manner when compared with DMSO‐treated bacteria (Figure 1a). The decrease was more pronounced at 180 min and compared significantly with ciprofloxacin, a reference antibiotic (Figure 1a–c). Furthermore, the decrease in absorbance was supported by reduction in CFU/ml of the organisms following incubation with the protocatechuic acid (Figure 2a–c).
Figure 1.

Viability of (a) Escherichia coli (ATCC 25922), (b) Pseudomonas aeruginosa (ATCC 27853), and (c) Staphylococcus aureus (ATCC 29213) exposed to protocatechuic acid (4× MIC). Values are mean ± SEM of three determinations and are statistically significant at p < .05
Figure 2.
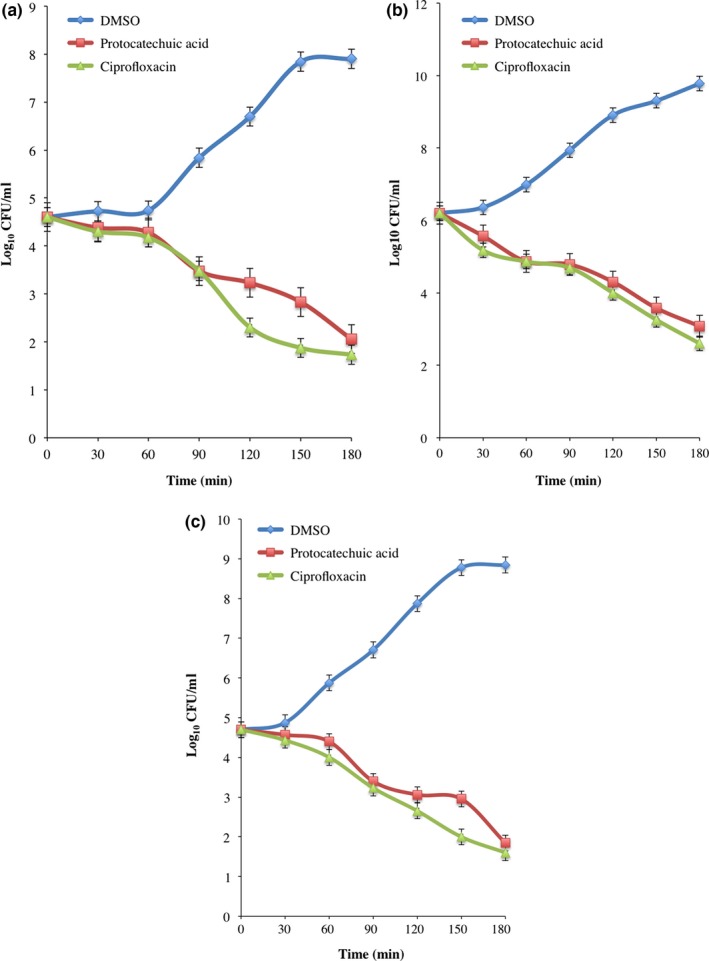
Viability (CFU/ml) of (a) Escherichia coli (ATCC 25922), (b) Pseudomonas aeruginosa (ATCC 27853), and (c) Staphylococcus aureus (ATCC 29213) exposed to protocatechuic acid (4× MIC). Values are mean ± SEM of three determinations and are statistically significant at p < .05
3.3. Oxidative stress biomarkers
The activities of superoxide dismutase and catalase increase (p < .05) in the cell‐free extracts of protocatechuic acid (4× MIC)‐treated E. coli, P. aeruginosa, and S. aureus when compared to DMSO‐treated organisms (Figure 3a–b). The increased activity of superoxide dismutase was 4.37‐, 7.90‐, and 10.11‐fold when compared to DMSO‐treated cells, while catalase activity increased by 3.85‐, 3.21‐, and 4.50‐fold.
Figure 3.

(a) Superoxide dismutase, (b) catalase, (c) reduced glutathione, (d) glutathione disulfide, and (e) NAD +/NADH of Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 29213) exposed to protocatechuic acid (4× MIC). Values are mean ± SEM of three determinations and are statistically significant at p < .05. *p < .05 vs DMSO. DMSO, dimethyl sulfoxide
The level of GSH in E. coli, P. aeruginosa, and S. aureus exposed to protocatechuic acid (4× MIC) decreased significantly (p < .05) by 84.16%, 66.39%, and 56.32%, respectively (Figure 3c). Conversely, GSSG increased significantly in the cell‐free extracts of protocatechuic acid‐treated organisms. This increase was compared significantly with ciprofloxacin‐treated organisms (Figure 3d). In addition, the NAD+/NADH ratio of cells exposed to protocatechuic acid increased by >1.5‐fold, indicating increased electron transport chain activity, which was compared with ciprofloxacin‐treated cells (Figure 3e).
Malondialdehyde, a product of lipid peroxidation, increased significantly (p < .05) by 8.56‐, 4.73‐, and 6.95‐fold, respectively, in the cell‐free extracts of E. coli, P. aeruginosa, and S. aureus treated with protocatechuic acid (4× MIC; Figure 4a). There was similar increase in the fragmented DNA of protocatechuic acid (4× MIC)‐treated organisms (Figure 4b).
Figure 4.

(a) Malondialdehyde and (b) fragmented DNA in protocatechuic acid‐treated Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 29213). Values are mean ± SEM of three determinations and are statistically significant at p < .05. *p < .05 vs DMSO. DMSO, dimethyl sulfoxide
3.4. Electron complex inhibition
The activity of electron transport complex I in protocatechuic acid‐treated E. coli, P. aeruginosa, and S. aureus decreased significantly (p < .05) when compared with DMSO‐treated cells (Figure 5a). Similarly, protocatechuic acid decreased the activity of complex II in E. coli, P. aeruginosa, and S. aureus when compared to DMSO‐treated cells (Figure 5b). Although ciprofloxacin produced a decrease in the activities of electron transport complexes I and II in E. coli, P. aeruginosa, and S. aureus, it did not compared with protocatechuic acid.
Figure 5.
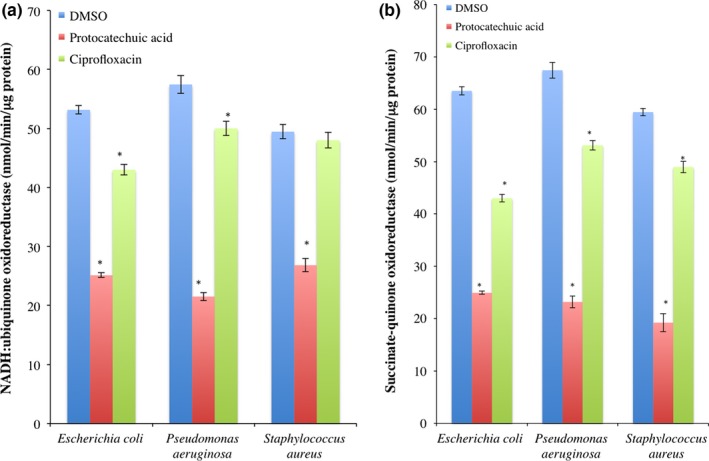
Activities of (a) NADH:ubiquinone oxidoreductase and (b) succinate:quinone oxidoreductase in Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 29213) exposed to protocatechuic acid (4× MIC). Values are mean ± SEM of three determinations and are statistically significant at p < .05. *p < .05 vs DMSO. DMSO, dimethyl sulfoxide
3.5. Involvement of reactive oxygen species
The involvement of reactive species in protocatechuic acid‐mediated bacterial lethality by checking the superoxide content and 2,2′‐bipyridyl inhibition of hydroxyl radical generation via fenton chemistry. Superoxide anion contents of E. coli, P. aeruginosa, and S. aureus increased significantly (p < .05) following protocatechuic acid (4× MIC) treatment when compared to the DMSO‐treated cells (Figure 6a). It produced 12.44‐, 27.34‐, and 16.29‐fold increase in superoxide contents of E. coli, P. aeruginosa, and S. aureus, respectively. Although ciprofloxacin produced similar increase in superoxide anion radical, it did not compared with protocatechuic acid.
Figure 6.

Involvement of reactive oxygen species in protocatechuic acid‐mediated bacterial death (a) superoxide anion radical content and viability (CFU/ml) of (b) Escherichia coli (ATCC 25922), (c) Pseudomonas aureginosa (ATCC 27853), and (d) Staphylococcus aureus (ATCC 29213) treated with protocatechuic acid (4× MIC) in the presence of 2,2′‐bipyridyl (500 μmol/L) and thiourea (150 mmol/L). Values are mean ± SEM of three determinations and are statistically significant at p < .05
There was significant increase in CFU/ml of E. coli, P. aeruginosa, and S. aureus treated with only 2,2′bipyridyl (500 μmol/L) and thiourea (150 mmol/L) when compared with protocatechuic acid‐treated organisms (Figure 6b–d). Although protocatechuic acid in combination with 2,2′bipyridyl or thiourea decreased the number of viable cells, it did not compare with organisms treated with only lophirone B and lophirone C (Figure 6b–d).
4. Discussion
The golden mean to healthy living is maintaining redox homeostasis, as any imbalance could result to the production of oxidized cellular macromolecules. In this study, we present the involvement of oxidative stress in protocatechuic acid‐mediated bacterial lethality.
Protocatechuic acid has been demonstrated to be potent antimicrobial agents with wide spectrum activity (Da‐Costa‐Rocha et al., 2014; Liu, Tsao, & Yin, 2005). The MIC and MBC values generated in this study are in consonance with previous studies, indicating that bacteriostatic and bactericidal activities of protocatechuic acid are dependent on the concentration. These values are supported by decrease in absorbance and CFU/ml for 3 hr incubation. Similar antibacterial activity of this compound was reported with the pure form and derivatives (Liu et al., 2005).
Imbalance in the antioxidant defense system and ROS generation leading to oxidative damage of cellular macromolecules such as proteins, lipids, and DNA have been implicated in antibiotics and antimicrobials mediated bacterial lethality (Ajiboye et al., 2016a,b; Dwyer et al., 2014; Lobritz et al., 2015; Samoilova, Smirnova, Muzyka, & Oktyabrsky, 2014; Wang, Zhao, Malik, & Drlica, 2010; Zhao & Drlica, 2014; Zhao, Hong, & Drlica, 2015). Generation of ROS (superoxide anion radical) have been demonstrated in the prooxidant activity of protocatechuic acid (Zeraik et al., 2014). The bacteria (E. coli, P. aeruginosa, and S. aureus) responded to this increase by enhancing the activity of SOD and CAT. This increase could be an attempt to detoxify elevated ROS (H2O2 and •O2 −).
Bacterial GSH complements cellular antioxidants by detoxifying H2O2 (Smirnova, Muzyka, & Oktyabrsky, 2012). Protocatechuic acid‐mediated depletion of GSH level in E. coli, P. aeruginosa, and S. aureus could perturb redox status and result to death. Although in a different cell culture, Babich, Sedletcaia, Kenigsberg, Babich, and Al (2002) reported similar GSH depletion by protocatechuic acid which is in consonance with our findings. Concomitant elevation in glutathione disulfide in protocatechuic acid‐treated bacteria evidently suggested the oxidation of GSH to GSSG. Consistent with our findings, esterified protocatechuic acid also led to the oxidation of GSH (Zeraik et al., 2014). The depletion of GSH and increased GSSG generation could distort redox balance resulting to oxidative attack on cellular macromolecules. Bactericidal agents have been reported to stimulate energy generating pathways such as citric acid cycle and electron transport leading to influx of electron into the electron transport chain through the reducing equivalent, NADH (Kohanski et al., 2007; Kohanski, Dwyer, & Collins, 2010). Indeed, protocatechuic enhanced influx of electron via NADH as evident from the increased NAD+/NADH ratio, which could result to electron and superoxide anion production.
Cellular macromolecules, DNA, and lipids are primary target of ROS leading to fragmentation of DNA and peroxidation of lipids (Ajiboye, 2010; Ajiboye et al., 2010). As such, products of lipid peroxidation and DNA fragmentation are useful indicator of oxidative status in bacteria. The increased level of MDA and fragmented DNA of protocatechuic acid‐treated bacteria is consistent with previous result obtained in human leukemia cells (Tseng et al., 2000) and cultured human cells from oral tissue (Babich et al., 2002). This increase indicates oxidative modification of cellular macromolecules in E. coli, P. aeruginosa, and S. aureus, possibly resulting from enhanced ROS generation.
Complexes I and III are large responsible for superoxide anion radical generation (Lanciano et al., 2013; Markevich & Hoek, 2015). Superoxide anion radical generated are dismutated to H2O2, which undergoes Fenton reaction (in the presence of Fe2+) to produce hydroxyl radical. Although the increased NAD+/NADH ratio evidently shows increased electron transport and correlate with superoxide anion radical generated. The inhibition of complexes I and II are indication that complex III and other sources may be responsible for the increased superoxide anion generated in this study.
Reactive oxygen species, in particular •O2 − and •OH−, have been implicated and documented as common mechanism for antimicrobials and antibiotics (Ajiboye and Haliru, 2016; Ajiboye et al., 2016b; Foti, Devadoss, Winkler, Collins, & Walker, 2012; Kohanski et al., 2007; Wang & Zhao, 2009).The elevated level of •O2 − in this study indicates enhanced ROS generation, which could have resulted via autoxidation of protocatechuic acid and inhibition of electron transport chain complexes. To further show the importance of reactive oxygen species generation in protocatechuic acid‐mediated bacterial lethality, E. coli, P. aeruginosa, and S, aureus were treated with protocatechuic acid and or 2,2′ dipyridyl (Fe chelator) and thiourea (hydroxyl radical) scavenger. Although the direct target of hydrogen peroxide is discerned, it undergoes Fenton reaction in the presence of Fe2+ to generate hydroxyl radical. Increase in CFU/ml of E. coli, P. aeruginosa, and S. aureus following incubation with protocatechuic acid in the presence of 2,2′ dipyridyl or thiourea indicates the involvement of hydroxyl radical in bacterial lethality. This supports previous studies that have established the involvement of hydroxyl radical as mechanism of antibacterial agents (Ajiboye et al., 2016b; Kohanski et al., 2007).
5. Conclusion
It is evident from the enhanced ROS generation, increased MDA and fragmented DNA, depleted reduced glutathione, and decreased respiratory chain activity that protocatechuic acid induced oxidative stress in its bacterial lethality against E. coli, P. aeruginosa, and S. aureus (Figure 7).
Figure 7.
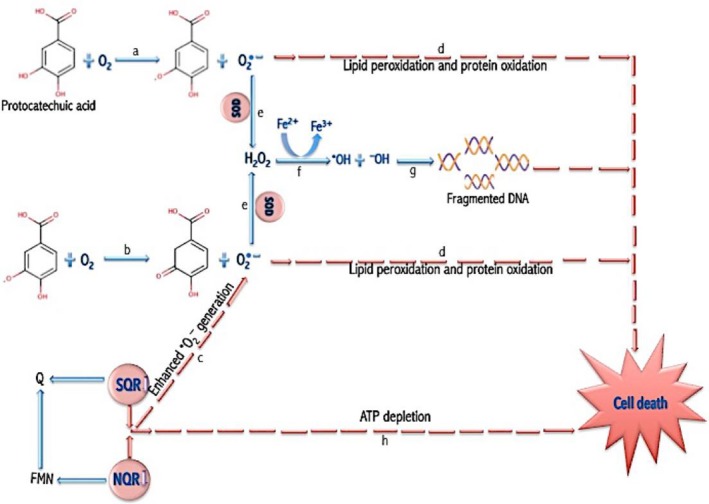
Proposed mechanism of protocatechuic acid‐mediated redox perturbation. (a) Autoxidation of protocatechuic acid generates •O2 − and semiquinone. (b) Semiquinone generated is further oxidized to generate more •O2 −. (c) The inhibition of NQR and SQR further enhanced •O2 − generation. (d) •O2 − attacks polyunsaturated fatty acid components of membrane (lipid peroxidation) and thiol group of protein (protein oxidation). (e) Superoxide dismutase also converts •O2 − to H2O2. (f) In the presence of Fe2+, hydrogen peroxide undergoes Fenton reaction leading to the generation of •OH. (g) •OH attacks DNA bases resulting to DNA fragmentation. (h) Inhibition of NQR and SQR also results in ATP depletion. These events, lipid peroxidation, protein oxidation, DNA fragmentation and ATP depletion, result to cell death. NQR, NADH:quinone oxidoreductase; SQR, succinate:quinone oxidoreductase; MDA, malondialdehyde; GSSG, glutathione disulfide; SOD, superoxide dismutase; CAT, catalase; GSH‐Px, glutathione peroxidase; GSH, reduced glutathione
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ajiboye TO, Habibu RS, Saidu K, et al. Involvement of oxidative stress in protocatechuic acid‐mediated bacterial lethality. MicrobiologyOpen. 2017;6:e472 https://doi.org/10.1002/mbo3.472
References
- Ajiboye, T. O. (2010). Redox status of the liver and kidney of 2,2‐dichlorovinyl dimethyl phosphate (DDVP) treated rats. Chemico‐Biological Interactions, 185, 202–207. [DOI] [PubMed] [Google Scholar]
- Ajiboye, T. O. , & Haliru, F. Z. (2016). Redox and respiratory chain related alterations in the bacterial lethality of lophirones B and C. Microbial Pathogenesis, 100, 95–111. [DOI] [PubMed] [Google Scholar]
- Ajiboye, T. O. , Mohammed, A. O. , Bello, S. A. , Yusuf, I. I. , Ibitoye, O. B. , Muritala, H. F. , & Onajobi, I. B. (2016a). Antibacterial activity of Syzygium aromaticum seed: Studies on oxidative stress biomarkers and membrane permeability. Microbial Pathogenesis, 95, 208–215. [DOI] [PubMed] [Google Scholar]
- Ajiboye, T. O. , Naibi, A. M. , Abdulazeez, I. O. , Alege, I. O. , Mohammed, A. O. , Bello, S. A. , … Muritala, H. F. (2016b). Involvement of oxidative stress in bactericidal activity of 2‐ (2‐ nitrovinyl) furan against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus . Microbial Pathogenesis, 91, 107–114. [DOI] [PubMed] [Google Scholar]
- Ajiboye, T. O. , Aliyu, M. , Isiaka, I. , Haliru, F. Z. , Ibitoye, O. B. , Uwazie, J. N. , … Mohammed, A. O. (2016c). Contribution of reactive oxygen species to (+)catechin‐mediated bacterial lethality. Chemico‐Biological Interactions, 258, 276–287. [DOI] [PubMed] [Google Scholar]
- Ajiboye, T. O. , Salau, A. K. , Yakubu, M. T. , Oladiji, A. T. , Akanji, M. A. , & Okogun, J. I. (2010). Aqueous extract of Securidaca longepedunculata root induce redox imbalance in male rat liver and kidney. Human and Experimental Toxicology, 29, 679–688. [DOI] [PubMed] [Google Scholar]
- Babich, H. , Sedletcaia, A. , Kenigsberg, B. , Babich, H. , & Al, E. T. (2002). In Vitro Cytotoxicity of Protocatechuic Acid to Cultured Human Cells from Oral Tissue : Involvement in Oxidative Stress. Pharmacology and Toxicology, 245–253. [DOI] [PubMed] [Google Scholar]
- Balouiri, M. , Sadiki, M. , & Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, K. (1956). A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochemical Journal, 62, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Liu, L. , Zhu, J. , Xu, B. , & Li, R. (2010). Effect of soybean oligosaccharides on blood lipid, glucose levels and antioxidant enzymes activity in high fat rats. Food Chemistry, 119, 1633–1636. [Google Scholar]
- Da‐Costa‐Rocha, I. , Bonnlaender, B. , Sievers, H. , Pischel, I. , & Heinrich, M . (2014). Hibiscus sabdariffa L. ‐ A phytochemical and pharmacological review. Food Chemistry, 164, 424–443. [DOI] [PubMed] [Google Scholar]
- Dixon, S. J. , & Stockwell, B. R. (2014). The role of iron and reactive oxygen species in cell death. Nature Chemical Biology, 10, 9–17. [DOI] [PubMed] [Google Scholar]
- Dwyer, D. J. , Belenky, P. A. , Yang, J. H. , MacDonald, I. C. , Martell, J. D. , Takahashi, N. , … Collins, J. J. (2014). Antibiotics induce redox‐related physiological alterations as part of their lethality. Proceedings of the National Academy of Sciences USA, 111, E2100–E2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70–77. [DOI] [PubMed] [Google Scholar]
- Fang, F. C . (2011). Antimicrobial actions of reactive oxygen species. MBio, 2, e00141–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, I. C. F. R. , Barros, L. , & Abreu, R. M. V. (2009). Antioxidants in wild mushrooms. Current Medicinal Chemistry, 16, 1543–1560. [DOI] [PubMed] [Google Scholar]
- Foti, J. J. , Devadoss, B. , Winkler, J. A. , Collins, J. J. , & Walker, G. C. (2012). Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science, 336, 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin, P. J. , & Hilf, R. (1976). A fluorometric method for determination of oxidized and reduced glutathione in tissues. Analytical Biochemistry, 74, 214–226. [DOI] [PubMed] [Google Scholar]
- Kohanski, M. A. , Dwyer, D. J. , & Collins, J. J. (2010). How antibiotics kill bacteria: from targets to networks. Nature Reviews Microbiology, 8, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski, M. A. , Dwyer, D. J. , Hayete, B. , Lawrence, C. A. , & Collins, J. J. (2007). A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell, 130, 797–810. [DOI] [PubMed] [Google Scholar]
- Lanciano, P. , Khalfaoui‐hassani, B. , Selamoglu, N. , Ghelli, A. , Rugolo, M. , & Daldal, F. (2013). Molecular mechanisms of superoxide production by complex III : A bacterial versus human mitochondrial comparative case study. Biochimica et Biophysica Acta (BBA)‐Bioenergetics, 1827, 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire, J. , Harrison, J. J. , & Turner, R. J. (2013). Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Publ. Gr., 11, 371–384. [DOI] [PubMed] [Google Scholar]
- Liu, K. Sen , Tsao, S. M. , & Yin, M. C. (2005). In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phyther. Res., 19, 942–945. [DOI] [PubMed] [Google Scholar]
- Lobritz, M. A. , Belenky, P. , Porter, C. B. M. , Gutierrez, A. , Yang, J. H. , Schwarz, E. G. , … Collins, J. J. (2015). Antibiotic efficacy is linked to bacterial cellular respiration. Proceedings of the National Academy of Sciences, 112, 8173–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevich, N. I. , & Hoek, J. B. (2015). Biochimica et Biophysica Acta Computational modeling analysis of mitochondrial superoxide production under varying substrate conditions and upon inhibition of different segments of the electron transport chain. BBA ‐ Bioenerg., 1847, 656–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra, H. P. , & Fridovich, I. (1972). The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. Journal of Biological Chemistry, 247, 3170–3175. [PubMed] [Google Scholar]
- Nickel, A. , Kohlhaas, M. , & Maack, C . (2014). Mitochondrial reactive oxygen species production and elimination. Journal of Molecular and Cellular Cardiology, 73, 26–33. [DOI] [PubMed] [Google Scholar]
- Reilly, C. A. , & Aust, S. D . (2001). Measurement of lipid peroxidation. Current Protocols in Toxicology, 2, 2.4.1–2.4.13. Chapter 2, Unit 2.4. [DOI] [PubMed] [Google Scholar]
- Samoilova, Z. , Smirnova, G. , Muzyka, N. , & Oktyabrsky, O. (2014). Medicinal plant extracts variously modulate susceptibility of Escherichia coli to different antibiotics ଝ. Microbiological Research, 169, 307–313. [DOI] [PubMed] [Google Scholar]
- Schweigert, N. , Zehnder, A. J. B. , & Eggen, R. I. L. (2001). Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environmental Microbiology, 3, 81–91. [DOI] [PubMed] [Google Scholar]
- Simić, A. , Manojlović, D. , Šegan, D. , & Todorović, M. (2007). Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules, 12, 2327–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova, G. , Muzyka, N. , & Oktyabrsky, O. (2012). Transmembrane glutathione cycling in growing Escherichia coli cells. Microbiological Research, 167, 166–172. [DOI] [PubMed] [Google Scholar]
- Storz, G. , & Imlay, J. A . (1999). Oxidative stress. Current Opinion in Microbiology, 2, 188–194. [DOI] [PubMed] [Google Scholar]
- Tseng, T. , Kao, T. , Chu, C. , Chou, F. , Lin, W. , & Wang, C. (2000). Induction of Apoptosis by Hibiscus Protocatechuic Acid in Human Leukemia Cells via Reduction of Retinoblastoma (RB) Phosphorylation and Bcl‐2 Expression. Biochemical Pharmacology, 60, 307–315. [DOI] [PubMed] [Google Scholar]
- Van Bergen, N. J. , Blake, R. E. , Crowston, J. G. , & Trounce, I. A. (2014). Mitochondrion Oxidative phosphorylation measurement in cell lines and tissues ☆. Mitochondrion, 15, 24–33. [DOI] [PubMed] [Google Scholar]
- Wang, X. , & Zhao, X. (2009). Contribution of oxidative damage to antimicrobial lethality. Antimicrobial Agents and Chemotherapy, 53, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Zhao, X. , Malik, M. , & Drlica, K. (2010). Contribution of reactive oxygen species to pathways of quinolone‐mediated bacterial cell death. Journal of Antimicrobial Chemotherapy, 65, 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, M.‐C. , Lin, C.‐C. , Wu, H.‐C. , Tsao, S.‐M. , & Hsu, C.‐K. (2009). Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. Journal of Agriculture and Food Chemistry, 57, 6468–6473. [DOI] [PubMed] [Google Scholar]
- Yip, E. C. H. , Chan, A. S. L. , Pang, H. , Tam, Y. K. , & Wong, Y. H. (2006). Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c‐Jun N‐terminal kinase‐dependent mechanism. Cell Biology and Toxicology, 22, 293–302. [DOI] [PubMed] [Google Scholar]
- Zeraik, M. L. , Petrônio, M. S. , Coelho, D. , Regasini, L. O. , Silva, D. H. S. , Da Fonseca, L. M. , … Ximenes, V. F. (2014). Improvement of pro‐oxidant capacity of protocatechuic acid by esterification. PLoS ONE, 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , & Drlica, K . (2014). Reactive oxygen species and the bacterial response to lethal stress. Current Opinion in Microbiology, 21, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Hong, Y. , & Drlica, K. (2015). Moving forward with reactive oxygen species involvement in antimicrobial lethality. Journal of Antimicrobial Chemotherapy, 70, 639–642. [DOI] [PMC free article] [PubMed] [Google Scholar]


