Abstract
Identifying the processes by which new phenotypes and species emerge has been a long‐standing effort in evolutionary biology. Young adaptive radiations provide a model to study patterns of morphological and ecological diversification in environmental context. Here, we use the recent radiation (ca. 12k years old) of the freshwater fish Arctic charr (Salvelinus alpinus) to identify abiotic and biotic environmental factors associated with adaptive morphological variation. Arctic charr are exceptionally diverse, and in postglacial lakes there is strong evidence of repeated parallel evolution of similar morphologies associated with foraging. We measured head depth (a trait reflecting general eco‐morphology and foraging ecology) of 1,091 individuals across 30 lake populations to test whether fish morphological variation was associated with lake bathymetry and/or ecological parameters. Across populations, we found a significant relationship between the variation in head depth of the charr and abiotic environmental characteristics: positively with ecosystem size (i.e., lake volume, surface area, depth) and negatively with the amount of littoral zone. In addition, extremely robust‐headed phenotypes tended to be associated with larger and deeper lakes. We identified no influence of co‐existing biotic community on Arctic charr trophic morphology. This study evidences the role of the extrinsic environment as a facilitator of rapid eco‐morphological diversification.
Keywords: adaptive morphology, Arctic charr, benthic–limnetic, ecological opportunity, environmental heterogeneity, freshwater fish, trophic morphology
1. INTRODUCTION
Identifying the environmental agents of natural selection has proven difficult because organisms live in environments that are profoundly complex, with multiple and potentially conflicting selection pressures. Some lineages, but not all, diversify rapidly in new environments, suggesting that a combination of extrinsic and intrinsic factors determine adaptive potential (Elmer, Lehtonen, Fan, & Meyer, 2013; Losos & Mahler, 2010; Stein, Gerstner, & Kreft, 2014). It is increasingly recognized that phenotypic change can arise surprisingly fast. This has been proven experimentally (Blount, Borland, & Lenski, 2008; Kawecki et al., 2012), through artificial selection such as crop modification and animal breeding (Conner, 2003; Meyer, DuVal, & Jensen, 2012; Neff & Rine, 2006), and shown in some naturally occurring populations as a response to diversifying selection (Elmer, Lehtonen, Kautt, Harrod, & Meyer, 2010; Franks, Sim, & Weis, 2007; Hendry, Nosil, & Rieseberg, 2007).
Rapid adaptive radiations across isolated islands and lakes are well recognized as important models for disentangling how diversity arises in nature, as they provide relatively simple replicated environments in which similar phenotypic diversity has arisen repeatedly (Elmer & Meyer, 2011; Gavrilets & Losos, 2009; Schluter, 2000). Some of the best known examples include Darwin's finches on the Galapagos Islands (Grant & Grant, 2011), Hawaiian silverswords (Baldwin, 1997), Anolis lizards on Caribbean islands (Losos, 2009), cichlid fishes inhabiting the Great African Rift (Turner, 2007) and Central American crater lakes (Recknagel, Elmer, & Meyer, 2014), and some northern postglacial fishes (Schluter, 2000).
Colonization and adaptation in postglacial lakes has occurred relatively recently, since the last retreat of the glaciers. Postglacial lakes can be characterized as rather simple, scalable, low productivity environments (Klemetsen, 2010). They usually support habitats comprising different foraging opportunities; for example, the littoral, a shallow water zone supporting relatively high benthic invertebrate productivity, and a limnetic zone supporting planktonic invertebrate production (Robinson & Wilson, 1994; Schluter, 2000). In addition to the spatial divergence of these foraging resources, the fishes also differ in characteristics related to how they access prey: benthivorous, planktivorous, and piscivorous fishes of many species differ substantially in morphologically functional traits (e.g., Schluter, 1993; Jonsson & Jonsson, 2001; Svanbäck & Eklöv, 2004; Kahilainen & Østbye, 2006; Fraser, Huntingford, & Adams, 2008; Garduño‐Paz & Adams 2010; Willacker, von Hippel, Wilton, & Walton, 2010). These traits have been well studied and are closely related to the different foraging strategies used in the respective habitats. Fishes inhabiting littoral benthic habitats have a diet consisting of macro‐invertebrates and are usually deeper bodied, with fewer gill rakers and a more robust head. In contrast, individuals tending to utilize the limnetic environment feed on plankton and are more elongate in body shape, have a higher number of gill rakers, and more slender heads (Jonsson & Jonsson, 2001; McPhail, 1994; Østbye et al., 2006). Postglacial fishes frequently show convergence and parallelisms in trophic morphology both within and across species (Elmer & Meyer, 2011; Schluter, 2000; Seehausen & Wagner, 2014). The most prominent examples of radiating postglacial fishes include threespine sticklebacks (Gasterosteus aculeatus) (McPhail, 1994), European and lake whitefish (Coregonus sp.) (Bernatchez et al., 2010; Østbye et al., 2006), lake trout (Salvelinus namaycush) (Chavarie, Howland, & Tonn, 2013; Muir, Hansen, Bronte, & Krueger, 2016), and Arctic charr (Salvelinus alpinus) (Garduño‐Paz, Adams, Verspoor, Knox, & Harrod, 2012; Jonsson & Jonsson, 2001).
Arctic charr in particular are regarded as one of the most variable vertebrates (Klemetsen, 2013). When colonizing lakes throughout the northern hemisphere, populations have diversified dramatically across lakes that differ in their bathymetry, surface area, and ecological features (Bush & Adams, 2007; Garduño‐Paz, Demetriou, & Adams, 2010; Garduño‐Paz et al., 2012; Woods et al., 2013). Across its geographical range, Arctic charr has repeatedly evolved discrete feeding specialists (Jonsson & Jonsson, 2001; Klemetsen, 2010; Kristjánsson et al., 2011; Schluter, 2000). These trophic morphs are associated with pronounced differences in body shape. Typically, littoral macro‐benthos feeding specialists have deeper bodies and express larger, more robust heads, with a blunt snout. In contrast, plankton feeding specialists tend to have a more delicate body and head shape with smaller terminal mouths, finer jaw structure, and usually larger eyes (Adams et al., 1998; Jonsson & Jonsson, 2001; Klemetsen et al., 2003; Knudsen, Amundsen, Klemetsen, & Soerensen, 2007; Skúlason, Snorrason, & Jonsson, 1999; Snorrason et al., 1994). Morphological differences between trophic morphs are functionally linked to alternative feeding strategies that are defined by attributes of the prey (Garduño‐Paz & Adams, 2010; Hooker et al., 2016; Malmquist, 1992). In particular, larger mouth gape allows feeding on larger prey, a link that has been experimentally shown in trophically polymorphic Arctic charr (Adams & Huntingford, 2002b). Freshwater fishes in general exhibit a strong functional link between gape size, head depth, and feeding strategy (Day & McPhail, 1996; Knudsen et al., 2007; Rüber & Adams, 2001). For example, in the famously trophically diverse Icelandic Arctic charr in Thingvallavatn a primary differentiation is in head morphology where all four morphs vary significantly in head shape traits and this is associated with specialization and segregation in diet (Snorrason et al., 1994).
Research to date on adaptive radiations has suggested that ecosystem size, ecological opportunity (e.g., number of available ecological niches), and intrinsic factors (such as phylogenetic constraints and sexual selection) are vital components of diversification (Elmer et al., 2013; Gavrilets & Losos, 2009; Losos, 2010; Wagner, Harmon, & Seehausen, 2012). However, these are relatively general drivers of adaptive radiation that are likely to manifest in different specific ways in different diverging lineages. For example, the size of the habitat occupied (e.g., the area of an island or lake) may increase ecological opportunity and has been shown to predict phenotypic and species diversity (Kisel & Barraclough, 2010; Losos & Schluter, 2000; Nosil & Reimchen, 2005; Ricklefs, 2007; Seehausen, 2006). Other studies on fishes found that lake depth is a better predictor for phenotypic diversification, particularly along the benthic–limnetic axis, for example in cichlid fishes (Recknagel et al., 2014; Wagner et al., 2012) or the radiations of postglacial fishes such as sticklebacks (Willacker et al., 2010), whitefishes (Vonlanthen et al., 2009), and Arctic charr (Alekseyev, Samusenok, Matveev, & Pichugin, 2002; Hindar & Jonsson, 1982). Bathymetric traits are often correlated because lakes that increase in area also tend to become deeper and therefore more voluminous (Gavrilets & Losos, 2009; Post, Pace, & Hairston, 2000). Therefore, bathymetric traits such as lake volume and surface area are most effectively summarized as ecosystem size (e.g., Fukami, 2004; Post et al., 2000; Reche, Pulido‐Villena, Morales‐Baquero, & Casamayor, 2005). In addition, research on fishes has shown that the biotic community, such as intraspecific abundance levels (Bolnick, 2004; Svanbäck & Bolnick, 2007) and interspecific interactions such as the number of competing species (Bourke, Magnan, & Rodríguez, 1999; Robinson, Wilson, Margosian, & Lotito, 1993; Vamosi, 2003) and predators (Vamosi, 2005), also influence diversification.
Identifying the underlying factor(s) driving the repeated diversification of postglacial fishes is crucial to help understand how distinct phenotypes evolve and to predict evolutionary outcomes across environmental scenarios. Using a large dataset of Arctic charr populations from postglacial lakes across the British Isles, we tested whether an ecologically relevant morphological character—relative head depth—is correlated with the bathymetric and ecological characteristics of lakes. Head depth is closely linked to functional feeding strategy and therefore directly and indirectly reflects the extensive ecomorphological variation of Arctic charr (Adams & Huntingford, 2002a,b; Adams, Woltering, & Alexander, 2003; Jonsson & Jonsson, 2001; Liem, 1993). We examined whether the average, extremes, and extent of variation in head morphology of a charr population could be predicted by the lake environment biotic and abiotic characteristics.
2. METHODS
Arctic charr (S. alpinus) were collected from across 30 lakes in Scotland and Ireland using Nordic survey gill nets (fish total N = 1,091; mean 36 fish per lake, range 10–82) (Figure 1a, Table S1). Gill nets consisted of 12 panels of differently sized mesh (5–55 mm knot to knot), 30 m long and 1.5 or 6 m deep, and are nonselective for Arctic charr in the size range of 45–495 mm (fork length) (Jensen & Hesthagen, 1996). A structured random sampling approach was used to ensure that all habitats within a lake were sampled (see Adams et al., 2006 for details); nets were set in the littoral, sublittoral, profundal, and pelagic zones (n = 4–14 nets per lake depending upon size).
Figure 1.
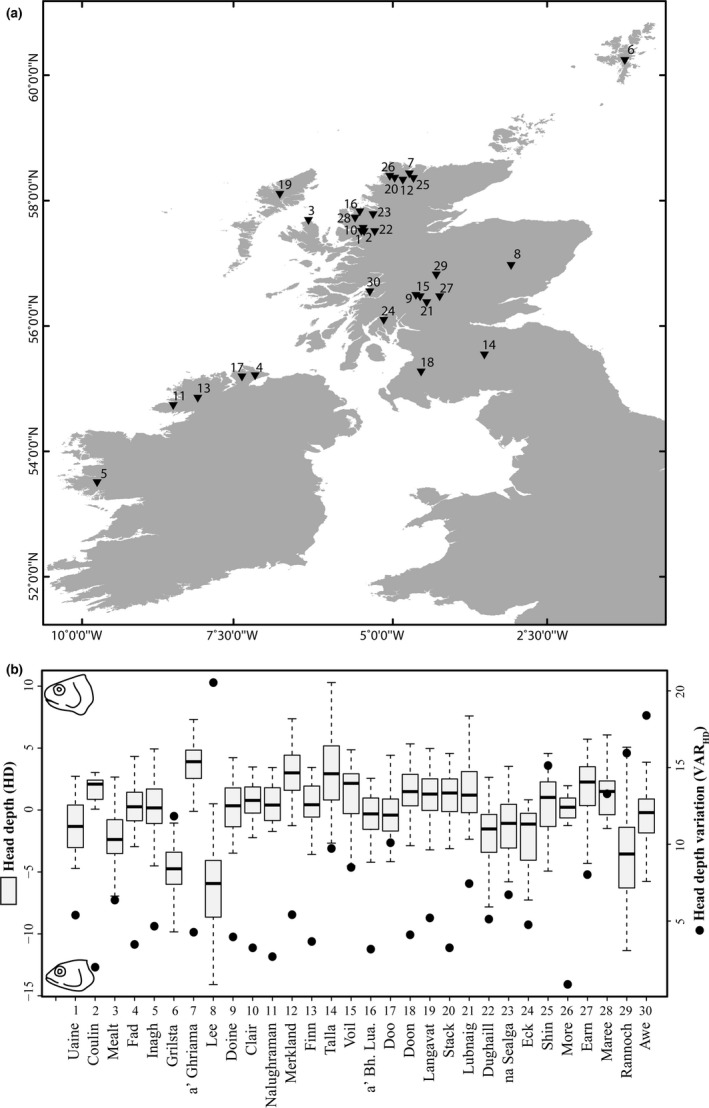
(a) Sampling sites of Arctic charr from the British Isles. Lakes are ranked according to their score on PC1 (or based on surface area in case they could not be included in the PCA), with low numbers indicating small, shallow, and species‐poor lakes while large numbers indicate greater surface area, deeper, and more species‐rich lakes. Lake names are listed with associated number in panel b. (b) Distribution of head depth is shown on the left y‐axis in gray boxes (black bar within box = median; whiskers = ± 1.5 IQR [outliers excluded for visualization]) and variation in head depth (VARHD) on the right y‐axis (black dots) for all individuals and across all lakes (total N = 1,091). Abbreviations: a’ Bh. Lua. = a’ Bhaid Luachraich
Head length, head depth, and fork length were measured for each adult individual collected. To correct for allometric effects, head depth and head length were regressed against fork length and we calculated the difference between the residuals in head depth and length for each individual; thus, a high value indicates a relatively deep‐headed individual, whereas a small value describes an individual with a shallow head relative to length. This univariate measure we use as an index for head depth (called HD hereafter) effectively describes functional ecomorphology (Adams et al., 1998) while minimizing data complexity. We calculated four morphological measures for each charr population: the average (MEANHD), maximum (MAXHD), and minimum (MINHD) head depth, and variance in head depth (VarHD) as a measure of morphological variation. All statistical analyses were carried out using R 3.0.2 (R Core Team 2013).
Alternatively, morphological metrics—in particular morphological variability—can be driven by demographic factors such that lakes with more individuals might exhibit greater variation in body shape, including head depth. We tested whether each of the four head depth measures depended on allelic richness, a genetic proxy for genetic diversity and population size. Estimates of microsatellite allelic richness were extracted from published literature (Wilson et al., 2004; six polymorphic nuclear microsatellite loci) on Arctic charr for 21 of the 30 lakes used in this present study (Table S1). In addition, we tested whether charr abundance had an effect on morphological traits. Abundance is an indicator for intraspecific competition within lakes and was recorded as catch per unit effort (CPUE), counted as the number of Arctic charr individuals caught per 100 m2 of net per 24 hr.
Environmental data were collated for each lake from published literature (Murray & Pullar, 1910), government agencies, and the authors’ own surveys (Table S1). The bathymetric parameters available were lake volume, average and maximum lake depth, lake surface area, and littoral zone area (defined as the area of the lake shallower than 4.5 m depth). The relevant biotic community parameters available were the number of competing species, number of predators, and total number of fish species in the lake community. Categorization as competitors or predators was based on the species’ ecology at adult stage. Competing species included: brown trout (Salmo trutta), Atlantic salmon (Salmo salar), roach (Rutilus rutilus), rainbow trout (Oncorhynchus mykiss), and powan (Coregonus lavaretus), fishes scored as predators were Northern pike (Esox lucius), European eel (Anguilla anguilla), European perch (Perca fluviatilis), and brown trout (Salmo trutta). Note that brown trout was both included as competitor and predator. European flounder (Platichthys flesus), brook lamprey (Lampetra planeri), minnow (Phoxinus phoxinus), and three‐spined stickleback (Gasterosteus aculeatus) were also included as part of the overall lake community (Table S1). Bathymetric parameters were normalized (log‐transformed). The percent of the lake substrate area categorized as littoral zone was arcsine transformed (Crawley, 2014). Ecological parameters (“biotic community”) were normalized using square root transformation (Crawley, 2014).
We used multiple lines of analysis to identify the associations among environmental characteristics and between those environmental characteristics and charr morphological variables MEANHD, MAXHD, MINHD, and VARHD.
First, we inferred whether predictor variables were correlated using Pearson's correlation coefficient (PCC) and principal component analysis (PCA). Based on those correlation estimates, we assigned predictors into three separate classes as described by the first three principal components of the PCA, each sharing a set of highly correlated variables: i) PC1 represents ecosystem size, with all lake size parameters (volume, surface area, maximum depth, and mean depth) as well as biotic community size having high positive loadings on this PC (thus high PC1 scores equate to large lakes); ii) PC2 mainly describes the biotic community with high negative loadings (thus high PC2 scores define lakes with depauperate biotic communities); and, iii) PC3 is associated with small lakes that have a small littoral area (thus high PC3 scores describe small, deep lakes with a steep shore gradient) (Tables S2 and S3, Figure S1).
Second, we performed multiple linear regressions to assess the relative contribution of predictor variables to each of the four morphological variables. Models were simplified by sequentially excluding nonsignificant parameters. The full model included five predictor variables: the first three principal components representing environmental and biotic lake characteristics, genetic diversity, and charr abundance (CPUE).
As an alternative approach to address multicollinearity, we used the relative importance test to assess the contribution of the individual lake characteristic variables, ranking them by model importance and accounting for collinearity between these correlated variables to estimate their relative importance (% of R 2) (Figure S2). The relative importance test “lmg” was used within the R package relaimpo, which averages sequential sums of squares over all orderings of predictor variables (Grömping, 2006). All four morphological variables were tested against all eight correlated predictor variables by implementing a bootstrapping algorithm (N = 1,000) to assess 95% confidence intervals.
3. RESULTS
3.1. Relationship among environmental variables
A number of lake environment characteristics were related, as inferred from the correlation estimates (PCCs; Table S2). Bathymetry traits (lake volume, surface area, maximum depth, and mean depth) were highly correlated. Proportion of littoral zone—an important feeding habitat for fishes—was negatively correlated with mean and maximum lake depth, showing that shallower lakes had relatively more littoral zone. Biotic community variables (fish community size, number of competing species, and number of predators) were highly correlated with each other and to a lesser extent also with ecosystem size or amount of littoral zone (Table S2).
PCA drew out similar relationships among environmental variables, with the amount of littoral zone loading in the opposite direction from ecosystem size on PC1, and biotic community distinct from bathymetric variables on PC2 (Figure S1). Overall PC1 captured a high proportion of variance (loadings all exceeded 0.3) for all abiotic and biotic variables. PC3 scores increased for lakes with a small surface area and small littoral zone but high volume (i.e., deep lakes relative to surface area). Overall, the first three axes explained 89.3% of the total variance (Table S3).
3.2. Eco‐morphology across populations
The Arctic charr populations differed dramatically in their head depth across lakes (Figure 1b). The most slender‐headed individual came from Loch Lee (MINHD of −16.91), and the individual with the bulkiest (deepest) heads were from Loch Awe (MAXHD of 25.45). MEANHD varied across lakes, from −7.06 in Loch Lee to 3.72 in a’ Ghriama.
A considerable number of Arctic charr populations showed high variability in head morphologies across lakes, with the most variability found in the populations of Loch Lee (VARHD of 20.54), followed by Loch Awe (VARHD of 18.40), Loch Rannoch (VARHD of 15.95), and Loch Shin (VARHD of 15.13) (Figure 1b). The lakes with low variability populations were dramatically less variable, for example, Loch More with VARHD of 0.87. The combined average variation for head depth (VARHD) across all lakes was 7.24.
This pattern of adaptively relevant morphological variability was not an effect of larger population size within lakes. We found that allelic richness was not associated with any of the four morphological variables in any model (Table 1). Hence, neutral genetic diversity did not have a significant effect on the variability nor in predicting the extent of trophic morphology. In addition, abundance of Arctic charr did not have an effect on any of the morphological traits, indicating that intraspecific competition did not significantly influence the degree of variation or extent of adaptive morphology.
Table 1.
Statistics for the best performing models (ecosystem size (=PC1), biotic community (=PC2), small, steep shore gradient, deep lakes (=PC3), genetic diversity, and charr abundance) and each morphological variable. Following model simplification, ecosystem size remained as the only significant parameter explaining morphology across all tests. Significant p‐values are shown in italics. Asterisks indicate significance levels, with *p < .05, **p < .01, and ***p < .001
| Morphology | Best model | Estimate | SE | t‐Value | p‐Value | R 2 |
|---|---|---|---|---|---|---|
| MEANHD | MEANHD ~ PC1 | −0.392 | 0.169 | −2.317 | .0297* | 0.189 |
| MINHD | MINHD ~ PC1 | −0.663 | 0.253 | −2.621 | .0153* | 0.230 |
| MAXHD | MAXHD ~ PC1 | 1.306 | 0.443 | 2.949 | .0070** | 0.274 |
| VARHD | VARHD ~ PC1 | 1.524 | 0.296 | 5.156 | <.0001*** | 0.536 |
3.3. Environment and the distribution of eco‐morphology
The average head morphology (MEANHD) of an Arctic charr population was significantly negatively associated with ecosystem size (p = .030, R 2 = 0.19, coefficient = −0.392; Figure 2a). These results show that charr populations with more slender heads are more likely to be found in lakes with a larger ecosystem size (greater surface area, deep, voluminous lakes), relatively smaller littoral zone, and a more complex fish community (Table 1, Figure 2a).
Figure 2.
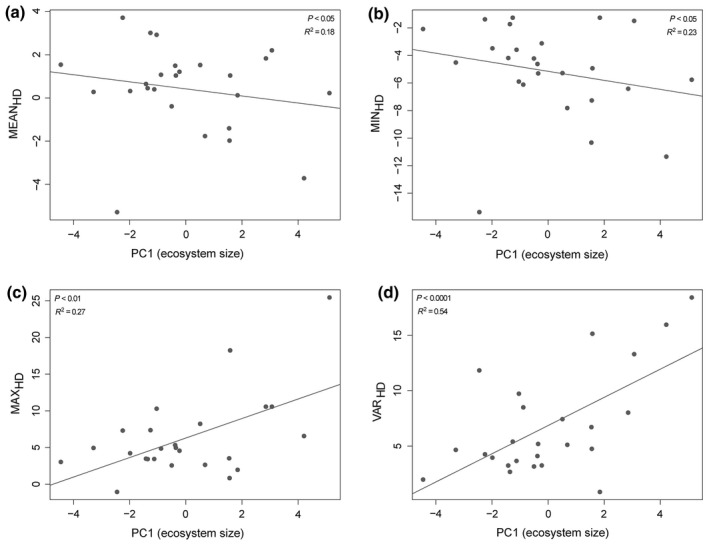
The relationship between Arctic charr morphological characteristics (a: MEANHD, b: MINHD, c: MAXHD, d: VARHD) and lake environment parameters (ecosystem size) that were significantly associated in linear regressions
The extremity of slender headedness (MINHD) for a population showed a significant negative association with ecosystem size (PC1) in the multiple linear regressions (p = .015, R 2 = 0.23, coefficient = −0.663; Table 1; Figure 2b). In agreement with the regression approach, in the relative importance tests littoral zone area was also identified as the highest contributing factor (% of R 2 = 32.3%, ΔR 2 to next best factor = 12.9%) (Figure S2). These results suggest that charr populations in shallower lakes are less extreme in MINHD, while larger lakes tend to support individuals that are more extremely slender headed.
The extremity of deep headedness (MAXHD) of a population was positively associated with ecosystem size (p = .007, R 2 = 0.27, coefficient = 1.306; Table 1; Figure 2c). This positive association remains as a trend even when excluding the most extreme MAXHD at highest ecosystem size (p = .266, R 2 = 0.06, coefficient = 0.416). Therefore, deep lakes with a greater surface area (larger ecosystem size) tend to support more extremely deep‐headed individuals. Similarly with the alternative relative importance approach, volume and surface area together explained more than half (59.6% of R 2) of the total variation in the correlation coefficient (R 2 = 0.58) between MAXHD and all predictive lake parameters (Figure S2).
In summary, the average head depth of an Arctic charr population generally decreased to be more slender with larger ecosystem size (i.e., great surface area, deep, and voluminous lakes). The extremes of head depth (MINHD and MAXHD) of the Arctic charr populations increased with ecosystem size, and this pattern was stronger for MAXHD compared to MINHD. There was no significant association between MEANHD, MINHD, or MAXHD with any of the principal components related to biotic community or to particularly small but deep lakes (Table 1).
3.4. Environment and the variability in eco‐morphology
The variation in head depth (VARHD) of a charr population was highly significantly associated with ecosystem size (p < .0001, R 2 = 0.54, coefficient = 1.524; Table 1; Figure 2d). Accordingly, with the relative importance approach volume and surface area explained more than 50% of the total R 2, with surface area being by far the largest contributor (32.1% of R 2) (Figure S2).
In summary, adaptively relevant morphological variation (VARHD) in Arctic charr significantly increased with greater surface area and deeper lakes, or larger ecosystem size. Ecological parameters alone (PC2: fish community complexity, number of predators, and number of competing species) did not have a significant effect on variation in head depth, nor did genetic diversity (allelic richness) or population abundance (CPUE).
4. DISCUSSION
We found that the mean, minimum, and maximum head depth of Arctic charr varied greatly across populations in different lakes, as did variability (Figure 1b). In freshwater fishes, head depth is well established to be closely associated with alternative ecomorphologies (Adams et al., 2003; Liem, 1991; Seehausen & Wagner, 2014). Regarding mean and maximum head depths, a bulkier head and blunt‐snouted morphology is strongly associated with littoral foraging in Arctic charr. In contrast, limnetic or pelagic fishes are more active foragers, adapted to high swimming velocity and more elongated head shape to enhance the ability to capture evasive prey as reflected in the distribution of minimum and mean head depths (Adams & Huntingford, 2002a,b; Adams et al., 1998; Hooker et al., 2016; Klemetsen, Knudsen, Primicerio, & Amundsen, 2006; Kristjánsson et al., 2011).
Here, we found that the degree of head depth variation in an Arctic charr population was significantly predicted by ecosystem size. In order of importance, lakes that had a greater surface area, a greater volume, were deeper, supported a larger fish community, and had a proportionately smaller littoral zone (i.e., steeper slopes) supported populations of Arctic charr with a more variable head shape (Table 1, Figure 2d). This pattern was not driven by demographic effects associated with the size or abundance of a lake's Arctic charr population, as shown by the lack of any significant relationship between head depth measures or variability, population genetic diversity and CPUE.
Our results are consistent with the proposal that larger environments with consequently greater complexity support more niches; this allows local adaptations to the more diverse range of available resources and has been found consistently across aquatic and terrestrial habitats (Gavrilets & Losos, 2009; Post et al., 2000; Tews et al., 2004). For example, lakes with greater surface area have been shown to support a higher degree of ecomorphological differentiation in sticklebacks (McPhail, 1993; Schluter & McPhail, 1992), brook charr (Bertrand, Marcogliese, & Magnan, 2008), European whitefish (Siwertsson et al., 2010), and African cichlid species richness (Salzburger & Meyer, 2004; Wagner, Harmon, & Seehausen, 2014). Lake depth in particular has been associated with trophically relevant variability along a benthic–limnetic gradient in several freshwater fishes, including an increase in morphological variation or sympatric differentiation in European whitefish ecomorphs in Scandinavia (Hayden, Harrod, & Kahilainen, 2014; Siwertsson et al., 2010) and in the Alpine region (Vonlanthen et al., 2009), in Neotropical crater lake cichlids (Recknagel et al., 2014), and in African great lake cichlids (Wagner et al., 2012). While it has been hypothesized that deeper lakes also increase the potential for the evolution of trophically variable and polymorphic Arctic charr populations (Alekseyev et al., 2002; Hindar & Jonsson, 1982), this has not been tested robustly. This is the first study to support the significance of lake depth in predicting adaptive diversity in Arctic charr.
Another important component of ecological opportunity is the paucity of co‐existing species (Robinson et al., 1993; Vamosi, 2003), as this may open ecological space and reduce resource competition within lakes (Schluter, 2000). Here, we find no significant effect of fish community complexity and no effect of the number of competing species on Arctic charr population‐level morphological variation, mean, or extremes (Table 1; Table S2). In several species of postglacial fishes, including stickleback (Vamosi, 2003), pumpkinseed sunfish (Robinson et al., 1993), and brook charr (Bourke et al., 1999), it has been shown that if ecological niches are already filled by a different but ecologically similar species, this might impede the evolution of intraspecific variation and polymorphism. It has also been shown that despite occupying similar depth habitats in allopatry, Arctic charr, perch, and whitefish can co‐exist with each other, exhibit different trophic polymorphisms, and occupy different habitats in sympatry (Hayden et al., 2014; Sandlund et al., 2010). In contrast, other studies found a relatively low effect of competing species on the evolution of intraspecific variability and polymorphism (Eloranta, Nieminen, & Kahilainen, 2015; Recknagel et al., 2014; Svanbäck, Eklöv, Fransson, & Holmgren, 2008). Our study suggests that interspecific competition might not always limit the ability of a species to diversify, even in low productivity postglacial lakes; rather, the trophic morphology of an Arctic charr population depends primarily on the abiotic environmental characteristic of ecosystem size.
The link between environmental parameters and the degree of trophic variability and polymorphism is not well understood. Previous research in stickleback and whitefish suggests that disruptive selection is strongest when environmental contrasts are stark (Bolnick & Lau, 2008; Landry, Vincent, & Bernatchez, 2007). In the 30 lakes we studied here, the relative proportion of limnetic zone increases with lake size and depth and the relative proportion of littoral zone decreases. Small lakes are generally dominated by the littoral zone, providing suitable habitat and resources for benthic foraging. With an increase in lake size and depth, the relative proportion of the limnetic zone increases, opening a new niche for Arctic charr to exploit. This has also been shown for Scandinavian Arctic charr, which shift their diet to a more limnetic source with increasing lake size (Eloranta, Kahilainen, et al., 2015). The Arctic charr populations studied here are more variable, but also more extreme in their head depth in lakes that are larger and have a more complex habitat available or increased ecological opportunity. Because head depth measures reflect trophic niche use in charr (Adams & Huntingford, 2002b; Smith & Skúlason, 1996), our findings suggest that more specialized morphologies can be found in greater surface area and deeper lakes. In several geographically distant and unconnected lakes, the presence of specialized deep‐water ecomorphologies has been reported in Arctic charr (e.g., Alekseyev et al., 2002; Hindar & Jonsson, 1982; Hooker et al., 2016), strengthening the importance of environment for such morphologies to evolve.
The high degree of variation in head depth might result from phenotypically plastic individuals or genetic differentiation of divergent phenotypes within a lake's population. Most likely, a combination of both mechanisms is contributing to the observed phenotypic variation, as has been reported previously in Arctic charr (Jonsson & Jonsson, 2001; Adams & Huntingford, 2004). Depending on the time of colonization, the number of colonization events and other extrinsic factors, different lake populations will vary in the degree of how specialized and genetically divergent they are. While we have focused on the overall individual‐ and population‐level variation and have not assessed morphological specializations in these charr populations, variation may reflect subtle polymorphic divergences in sympatry (e.g., Adams et al., 1998; Garduño‐Paz et al., 2012). We did not find an effect of intraspecific competition and genetic diversity on the overall morphological variation; however, these factors likely have an impact on whether divergent ecomorphologies become initially established. For example, when conditions are stable over time (Taylor et al., 2006; Vonlanthen et al., 2012) and strong intraspecific competition prevails (Bolnick, 2004; Svanbäck & Bolnick, 2007), differentiation between individuals might become genetically fixed. This can be facilitated by differences in spawning time, spawning location, and habitat use that increases trophic variability, as has been evidenced repeatedly in Arctic charr (Adams et al., 1998, 2006; Jonsson & Jonsson, 2001). Our results suggest that environmental heterogeneity and consequently selection for phenotypic extremes in charr are strongest in deep lakes that are not dominated by littoral zone. This has the important implication that external context such as environment, rather than population‐specific intrinsic factors such as genetics (Elmer, 2016), determine the trophic variability and potential for adaptive diversification in these fishes.
5. CONCLUSION
We find that ecosystem size has a significant impact on adaptive morphological variation in an extensive diversification of Arctic charr. The most extreme head depths are found in larger lakes and the sympatric variation in head depth of a charr population significantly increased with lake ecosystem size. This adaptive morphological variation translates to high levels of extant diversity and may facilitate the formation of ecological specialists. The extent to which these diversifications are promoted by intrinsic factors such as genetic diversity vs. extrinsic factors such as environmental characteristics is still debated. Our findings suggest that ecological opportunities available through larger ecosystems (greater surface area, deeper, and more voluminous lakes) are the most significant component for this stereotypical diversification in a temperate radiation of freshwater fishes.
CONFLICT OF INTEREST
None declared.
Supporting information
ACKNOWLEDGMENTS
We would like to thank F. Huntingford, A. Grant, A. Lyle, M. Hughes, G. Alexander, N. Bissett, A. Duguid, R. Wilson, and P. Cunningham for supporting and/or assisting fieldwork. Bathymetric data for Irish loughs were kindly provided by the Environmental Protection Agency. We would like to thank P. Johnson for advice on statistical analyses and N. O'Hanlon for assistance with mapping. This research was funded by a Marie Curie CIG (grant no. 321999) to KRE, INTERREG IVA (project 2859 ‘IBIS’) to CEA, and a Leonardo da Vinci training grant to HR.
Recknagel H, Hooker OE, Adams CE, Elmer KR. Ecosystem size predicts eco‐morphological variability in a postglacial diversification. Ecol Evol. 2017;7:5560–5570. https://doi.org/10.1002/ece3.3013
REFERENCES
- Adams, C. E. , Fraser, D. , Huntingford, F. A. , Greer, R. B. , Askew, C. M. , & Walker, A. F. (1998). Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. Journal of Fish Biology, 52, 1259–1271. [Google Scholar]
- Adams, C. E. , & Huntingford, F. A. (2004). Incipient speciation driven by phenotypic plasticity? Evidence from sympatric populations of Arctic charr. Biological Journal of the Linnean Society, 81(4), 611–618. [Google Scholar]
- Adams, C. E. , Hamilton, D. J. , Mccarthy, I. , Wilson, A. J. , Grant, A. , Alexander, G. , … Skúlason, S. (2006). Does breeding site fidelity drive phenotypic and genetic sub‐structuring of a population of Arctic charr? Evolutionary Ecology, 20, 11–26. [Google Scholar]
- Adams, C. E. , & Huntingford, F. A. (2002a). Inherited differences in head allometry in polymorphic Arctic charr from Loch Rannoch, Scotland. Journal of Fish Biology, 60, 515–520. [Google Scholar]
- Adams, C. E. , & Huntingford, F. A. (2002b). The functional significance of inherited differences in feeding morphology in a sympatric polymorphic population of Arctic charr. Evolutionary Ecology, 16, 15–25. [Google Scholar]
- Adams, C. E. , Woltering, C. , & Alexander, G. (2003). Epigenetic regulation of trophic morphology through feeding behaviour in Arctic charr, Salvelinus alpinus . Biological Journal of the Linnean Society, 78, 43–49. [Google Scholar]
- Alekseyev, S. S. , Samusenok, V. P. , Matveev, A. N. , & Pichugin, M. Y. (2002). Diversification, sympatric speciation, and trophic polymorphism of arctic charr, Salvelinus alpinus complex, in Transbaikalia. Environmental Biology of Fishes, 64, 97–114. [Google Scholar]
- Baldwin, B. (1997). Adaptive radiation of the Hawaiian silversword alliance: Congruence and conflict of phylogenetic evidence from molecular and non‐molecular investigations In Givnish T. J., & Sytsma K. J. (Eds.), Molecular evolution and adaptive radiation (pp. 103–128). Cambridge, UK: Cambridge Univ. Press. [Google Scholar]
- Bernatchez, L. , Renaut, S. , Whiteley, A. R. , Derome, N. , Jeukens, J. , & Landry, L. (2010). On the origin of species: insights from the ecological genomics of the lake whitefish. Philosophical Transactions of the Royal Society of London. Series B, 365, 1783–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, M. , Marcogliese, D. J. , & Magnan, P. (2008). Trophic polymorphism in brook charr revealed by diet, parasites and morphometrics. Journal of Fish Biology, 72, 555–572. [Google Scholar]
- Blount, Z. D. , Borland, C. Z. , & Lenski, R. E. (2008). Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli . Proceedings of the National Academy of Sciences of the United States of America, 105, 7899–7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick, D. I. (2004). Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution, 58, 608–618. [PubMed] [Google Scholar]
- Bolnick, D. I. , & Lau, O. L. (2008). Predictable patterns of disruptive selection in stickleback in postglacial lakes. American Naturalist, 172, 1–11. [DOI] [PubMed] [Google Scholar]
- Bourke, P. , Magnan, P. , & Rodríguez, M. A. (1999). Phenotypic responses of lacustrine brook char in relation to the intensity of interspecific competition. Evolutionary Ecology, 13, 19–31. [Google Scholar]
- Bush, V. , & Adams, C. E. (2007). Using phenotypic variation to determine conservation value: Application of a novel approach to Arctic charr. Ecology of Freshwater Fish, 16, 29–33. [Google Scholar]
- Chavarie, L. , Howland, K. L. , & Tonn, W. M. (2013). Sympatric polymorphism in Lake Trout: The coexistence of multiple shallow‐water morphotypes in Great Bear Lake. Transactions of the American Fisheries Society, 142, 814–823. [Google Scholar]
- Conner, J. K. (2003). Artificial selection: A powerful tool for ecologists. Ecology, 84, 1650–1660. [Google Scholar]
- Crawley, M. J. (2014). The R book. New York, NY: John Wiley & Sons. [Google Scholar]
- Day, T. , & McPhail, J. D. (1996). The effect of behavioural and morphological plasticity on foraging efficiency in the threespine stickleback (Gasterosteus sp.). Oecologia, 108, 380–388. [DOI] [PubMed] [Google Scholar]
- Elmer, K. R. (2016). Genomic tools for new insights to variation, adaptation, and evolution in the salmonid fishes: A perspective for charr. Hydrobiologia [online early], 1–18. https://doi.org/10.1007/s10750-015-2614-5 [Google Scholar]
- Elmer, K. R. , Lehtonen, T. K. , Fan, S. , & Meyer, A. (2013). Crater lake colonization by Neotropical cichlid fishes. Evolution, 67, 281–288. [DOI] [PubMed] [Google Scholar]
- Elmer, K. R. , Lehtonen, T. K. , Kautt, A. F. , Harrod, C. , & Meyer, A. (2010). Rapid sympatric ecological differentiation of crater lake cichlid fishes within historic times. BMC Biology, 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, K. R. , & Meyer, A. (2011). Adaptation in the age of ecological genomics: Insights from parallelism and convergence. Trends in Ecology & Evolution, 26, 298–306. [DOI] [PubMed] [Google Scholar]
- Eloranta, A. P. , Kahilainen, K. K. , Amundsen, P. A. , Knudsen, R. , Harrod, C. , & Jones, R. I. (2015). Lake size and fish diversity determine resource use and trophic position of a top predator in high‐latitude lakes. Ecology and Evolution, 5, 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta, A. P. , Nieminen, P. , & Kahilainen, K. K. (2015). Trophic interactions between introduced lake trout (Salvelinus namaycush) and native Arctic charr (S. alpinus) in a large Fennoscandian subarctic lake. Ecology of Freshwater Fish, 24, 181–192. [Google Scholar]
- Franks, S. J. , Sim, S. , & Weis, A. E. (2007). Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences of the United States of America, 104, 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, D. , Huntingford, F. A. , & Adams, C. E. (2008). Foraging specialisms, prey size and life‐history patterns: A test of predictions using sympatric polymorphic Arctic charr (Salvelinus alpinus). Ecology of Freshwater Fish, 17, 1–9. [Google Scholar]
- Fukami, T. (2004). Assembly history interacts with ecosystem size to influence species diversity. Ecology, 85, 3234–3242. [Google Scholar]
- Garduño‐Paz, M. V. , & Adams, C. E. (2010). Discrete prey availability promotes foraging segregation and early divergence in Arctic charr, Salvelinus alpinus . Hydrobiologia, 650, 15–26. [Google Scholar]
- Garduño‐Paz, M. V. , Adams, C. E. , Verspoor, E. , Knox, D. , & Harrod, C. (2012). Convergent evolutionary processes driven by foraging opportunity in two sympatric morph pairs of Arctic charr with contrasting post‐glacial origins. Biological Journal of the Linnean Society, 106, 794–806. [Google Scholar]
- Garduño‐Paz, M. V. , Demetriou, M. , & Adams, C. E. (2010). Variation in scale shape among alternative sympatric phenotypes of Arctic charr Salvelinus alpinus from two lakes in Scotland. Journal of Fish Biology, 76, 1491–1497. [DOI] [PubMed] [Google Scholar]
- Gavrilets, S. , & Losos, J. B. (2009). Adaptive radiation: Contrasting theory with data. Science, 323, 732–737. [DOI] [PubMed] [Google Scholar]
- Grant, P. R. , & Grant, B. R. (2011). How and why species multiply: The radiation of Darwin's finches. Princeton, NJ: Princeton Univ. Press. [Google Scholar]
- Grömping, U. (2006). Relative importance for linear regression in R: The package relaimpo. Journal of Statistical Software, 17, 1–27. [Google Scholar]
- Hayden, B. , Harrod, C. , & Kahilainen, K. K. (2014). Lake morphometry and resource polymorphism determine niche segregation between cool‐ and cold‐water‐adapter fish. Ecology, 95, 538–552. [DOI] [PubMed] [Google Scholar]
- Hendry, A. P. , Nosil, P. , & Rieseberg, L. H. (2007). The speed of ecological speciation. Functional Ecology, 21, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindar, K. , & Jonsson, B. (1982). Habitat and food segregation of dwarf and normal Arctic charr (Salvelinus alpinus) from Vangsvatnet Lake, western Norway. Canadian Journal of Fisheries and Aquatic Science, 39, 1030–1045. [Google Scholar]
- Hooker, O. E. , Barry, J. , Van Leeuwen, T. E. , Lyle, A. , Newton, J. , Cunningham, P. , & Adams, C. E. (2016). Morphological, ecological and behavioural differentiation of sympatric profundal and pelagic Arctic charr (Salvelinus alpinus) in Loch Dughaill Scotland. Hydrobiologia, 783, 209–221. [Google Scholar]
- Jensen, J. W. , & Hesthagen, T. (1996). Direct estimates of the selectivity of a multimesh and a series of single gillnets for brown trout. Journal of Fish Biology, 49, 33–40. [Google Scholar]
- Jonsson, B. , & Jonsson, N. (2001). Polymorphism and speciation in Arctic charr. Journal of Fish Biology, 58, 605–638. [Google Scholar]
- Kahilainen, K. K. , & Østbye, K. (2006). Morphological differentiation and resource polymorphism in three sympatric whitefish Coregonus lavaretus (L.) forms in a subarctic lake. Journal of Fish Biology, 68, 63–79. [Google Scholar]
- Kawecki, T. J. , Lenski, R. E. , Ebert, D. , Hollis, B. , Olivieri, I. , & Whitlock, M. C. (2012). Experimental evolution. Trends in Ecology & Evolution, 27, 547–560. [DOI] [PubMed] [Google Scholar]
- Kisel, Y. , & Barraclough, T. G. (2010). Speciation has a spatial scale that depends on levels of gene flow. American Naturalist, 175, 316–334. [DOI] [PubMed] [Google Scholar]
- Klemetsen, A. (2010). The charr problem revisited: Exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshwater Reviews, 3, 49–74. [Google Scholar]
- Klemetsen, A. (2013). The most variable vertebrate on Earth. Journal of Ichthyology, 53, 781–791. [Google Scholar]
- Klemetsen, A. , Amundsen, P.‐A. , Dempson, J. B. , Jonsson, B. , Jonsson, N. , O'Connell, M. F. , & Mortensen, E. (2003). Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish, 12, 1–59. [Google Scholar]
- Klemetsen, A. , Knudsen, R. , Primicerio, R. , & Amundsen, P.‐A. (2006). Divergent, genetically based feeding behaviour of two sympatric Arctic charr, Salvelinus alpinus (L.), morphs. Ecology of Freshwater Fish, 15, 350–355. [Google Scholar]
- Knudsen, R. , Amundsen, P.‐A. , Klemetsen, R. A. , & Soerensen, P. (2007). Contrasting niche‐based variation in trophic morphology within Arctic charr populations. Evolutionary Ecology Research, 9, 1005–1021. [Google Scholar]
- Kristjánsson, B. K. , Malmquist, H. J. , Ingimarsson, F. , Antonsson, T. , Snorrason, S. S. , & Skúlason, S. (2011). Relationships between lake ecology and morphological characters in Icelandic Arctic charr, Salvelinus alpinus . Biological Journal of the Linnean Society, 103, 761–771. [Google Scholar]
- Landry, L. , Vincent, W. F. , & Bernatchez, L. (2007). Parallel evolution of lake whitefish dwarf ecotypes in association with limnological features of their adaptive landscape. Journal of Evolutionary Biology, 20, 971–984. [DOI] [PubMed] [Google Scholar]
- Liem, K. F. (1991). A functional approach to the development of the head of teleosts: Implications on constructional morphology and constraints In Schmidt‐Kittler N., & Vogel K. (Eds.), Constructional morphology and evolution (pp. 231–249). Berlin, Heidelberg Germany: Springer. [Google Scholar]
- Liem, K. F. (1993). Ecomorphology of the teleostean skull In Hanken J., & Hall B. K. (Eds.), The skull (pp. 422–452). Chicago, IL: Univ. of Chicago Press. [Google Scholar]
- Losos, J. B. (2009). Lizards in an evolutionary tree: Ecology and adaptive radiation of anoles. Berkeley, CA: Univ. of California Press. [Google Scholar]
- Losos, J. B. (2010). Adaptive radiation, ecological opportunity, and evolutionary determinism. American Naturalist, 175, 623–639. [DOI] [PubMed] [Google Scholar]
- Losos, J. B. , & Mahler, D. L. (2010). Adaptive radiation: the interaction of ecological opportunity, adaptation and speciation. In Bell M. A., Futuyma D. J., Eanes W. F., & Levinton J. S. (Eds.), Evolution Since Darwin: The first 150 years. Sunderland, USA: Sinauer Associates. [Google Scholar]
- Losos, J. B. , & Schluter, D. (2000). Analysis of an evolutionary species–area relationship. Nature, 408, 847–850. [DOI] [PubMed] [Google Scholar]
- Malmquist, H. J. (1992). Phenotype‐specific feeding behaviour of two arctic charr Salvelinus alpinus morphs. Oecologia, 92, 354–361. [DOI] [PubMed] [Google Scholar]
- McPhail, J. D. (1993). Ecology and evolution of sympatric sticklebacks (Gasterosteus): Origin of the species pairs. Canadian Journal of Zoology, 71, 515–523. [Google Scholar]
- McPhail, J. D. (1994). Speciation and the evolution of reproductive isolation in the sticklebacks (Gasterosteus) of south‐western British Columbia In Bell M., & Foster S. (Eds.), The evolutionary biology of the threespine stickleback (pp. 399–437). Oxford, UK: Oxford Univ. Press. [Google Scholar]
- Meyer, R. S. , DuVal, A. E. , & Jensen, H. R. (2012). Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytologist, 196, 29–48. [DOI] [PubMed] [Google Scholar]
- Muir, A. M. , Hansen, M. J. , Bronte, C. R. , & Krueger, C. C. (2016). If Arctic charr Salvelinus alpinus is ‘the most diverse vertebrate’, what is the lake charr Salvelinus namaycush? Fish and Fisheries, 17, 1194–1207. [Google Scholar]
- Murray, J. , & Pullar, L. (1910). Bathymetrical survey of the Scottish fresh‐water lochs (Vols. I‐VI). Edinburgh, UK: Challenger office. [Google Scholar]
- Neff, M. W. , & Rine, J. (2006). A fetching model organism. Cell, 124, 229–231. [DOI] [PubMed] [Google Scholar]
- Nosil, P. , & Reimchen, T. E. (2005). Ecological opportunity and levels of morphological variance within freshwater stickleback populations. Biological Journal of the Linnean Society, 86, 297–308. [Google Scholar]
- Østbye, K. P. A. , Bernatchez, A. L. , Klemetsen, A. , Knudsen, R. , Kristoffersen, R. , Naesje, T. F. , & Hindar, K. (2006). Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times. Molecular Ecology, 15, 3983–4001. [DOI] [PubMed] [Google Scholar]
- Post, D. M. , Pace, M. L. , & Hairston, N. G. (2000). Ecosystem size determines food‐chain length in lakes. Nature, 405, 1047–1049. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org/ [Google Scholar]
- Reche, I. , Pulido‐Villena, E. , Morales‐Baquero, R. , & Casamayor, E. O. (2005). Does ecosystem size determine aquatic bacterial richness? Ecology, 86, 1715–1722. [Google Scholar]
- Recknagel, H. , Elmer, K. R. , & Meyer, A. (2014). Crater lake habitat predicts morphological diversity in adaptive radiations of cichlid fishes. Evolution, 68, 2145–2155. [DOI] [PubMed] [Google Scholar]
- Ricklefs, R. E. (2007). History and diversity: Explorations at the intersection of ecology and evolution. American Naturalist, 170, S56–S70. [DOI] [PubMed] [Google Scholar]
- Robinson, B. W. , & Wilson, D. S. (1994). Character release and displacement in fishes: A neglected literature. American Naturalist, 144, 596–627. [Google Scholar]
- Robinson, B. W. , Wilson, D. S. , Margosian, A. S. , & Lotito, P. T. (1993). Ecological and morphological differentiation by pumpkinseed sunfish in lakes without bluegill sunfish. Evolutionary Ecology, 7, 451–464. [Google Scholar]
- Rüber, L. , & Adams, D. C. (2001). Evolutionary convergence of body shape and trophic morphology in cichlids from Lake Tanganyika. Journal of Evolutionary Biology, 14, 325–332. [Google Scholar]
- Salzburger, W. , & Meyer, A. (2004). The species flocks of East African cichlid fishes: Recent advances in molecular phylogenetics and population genetics. Naturwissenschaften, 91, 277–290. [DOI] [PubMed] [Google Scholar]
- Sandlund, O. T. , Museth, J. , Næsje, T. F. , Rognerud, S. , Saksgård, R. , Hesthagen, T. , & Borgstrøm, R. (2010). Habitat use and diet of sympatric Arctic charr (Salvelinus alpinus) and whitefish (Coregonus lavaretus) in five lakes in southern Norway: Not only interspecific population dominance? Hydrobiologia, 650, 27–41. [Google Scholar]
- Schluter, D. (1993). Adaptive radiation in sticklebacks: Size, shape, and habitat use efficiency. Ecology, 74, 699–709. [Google Scholar]
- Schluter, D. (2000). The ecology of adaptive radiations. New York, NY: Oxford University Press. [Google Scholar]
- Schluter, D. , & McPhail, J. D. (1992). Ecological character displacement and speciation in sticklebacks. American Naturalist, 140, 85–108. [DOI] [PubMed] [Google Scholar]
- Seehausen, O. (2006). African cichlid fish: A model system in adaptive radiation research. Proceedings of the Royal Society of London, Series B: Biological Sciences, 273, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen, O. , & Wagner, C. E. (2014). Speciation in freshwater fishes. Annual Review of Ecology Evolution and Systematics, 45, 621–651. [Google Scholar]
- Siwertsson, A. , Knudsen, R. , Kahilainen, K. K. , Praebel, K. , Primicerio, R. , & Amundsen, P.‐A. (2010). Sympatric diversification as influenced by ecological opportunity and historical contingency in a young species lineage of whitefish. Evolutionary Ecology Research, 12, 929–947. [Google Scholar]
- Skúlason, S. , Snorrason, S. S. , & Jonsson, B. (1999). Sympatric morphs, populations and speciation in freshwater fish with emphasis on Arctic charr In Magurran A. E., & May R. M. (Eds.), Evolution of biological diversity (pp. 70–92). New York, NY: Oxford Press. [Google Scholar]
- Smith, T. B. , & Skúlason, S. (1996). Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics, 27, 111–133. [Google Scholar]
- Snorrason, S. S. , Skúlason, S. S. , Jonsson, B. , Malmquist, H. J. , Jonasson, P. M. , Sandlund, O. T. , & Lindem, T. (1994). Trophic specialisation in Arctic charr Salvelinus alpinus (Pisces: Salmonidae): Morphological divergence and ontogenetic niche shifts. Biological Journal of the Linnean Society, 51, 1–18. [Google Scholar]
- Stein, A. , Gerstner, K. , & Kreft, H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters, 17, 866–880. [DOI] [PubMed] [Google Scholar]
- Svanbäck, R. , & Bolnick, D. I. (2007). Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society of London, Series B: Biological Sciences, 274, 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanbäck, R. , & Eklöv, P. (2004). Morphology in perch affects habitat specific feeding efficiency. Functional Ecology, 18, 503–510. [Google Scholar]
- Svanbäck, R. , Eklöv, P. , Fransson, R. , & Holmgren, K. (2008). Intraspecific competition drives multiple species resource polymorphism in fish communities. Oikos, 117, 114–124. [Google Scholar]
- Taylor, E. B. , Boughman, J. W. , Groenenboom, M. , Sniatynski, M. , Schluter, D. , & Gow, J. L. (2006). Speciation in reverse: Morphological and genetic evidence of the collapse of a three‐spined stickleback (Gasterosteus aculeatus) species pair. Molecular Ecology, 15, 343–355. [DOI] [PubMed] [Google Scholar]
- Tews, J. , Brose, U. , Grimm, V. , Tielborger, K. , Wichmann, M. C. , & Schwager, M. (2004). Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. Journal of Biogeography, 31, 79–92. [Google Scholar]
- Turner, G. F. (2007). Adaptive radiation of cichlid fish. Current Biology, 17, R827–R831. [DOI] [PubMed] [Google Scholar]
- Vamosi, S. M. (2003). The presence of other fish species affects speciation in threespine sticklebacks. Evolutionary Ecology Research, 5, 717–730. [Google Scholar]
- Vamosi, S. M. (2005). On the role of enemies in divergence and diversification of prey: A review and synthesis. Canadian Journal of Zoology, 83, 894–910. [Google Scholar]
- Vonlanthen, P. , Bittner, D. , Hudson, A. G. , Young, K. A. , Müller, R. , Lundsgaard‐Hansen, B. , … Seehausen, O. (2012). Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature, 482, 357–362. [DOI] [PubMed] [Google Scholar]
- Vonlanthen, P. , Roy, D. , Hudson, A. G. , Largiader, C. R. , Bittner, D. , & Seehausen, O. (2009). Divergence along a steep ecological gradient in lake whitefish (Coregonus sp.). Journal of Evolutionary Biology, 22, 498–514. [DOI] [PubMed] [Google Scholar]
- Wagner, C. E. , Harmon, L. J. , & Seehausen, O. (2012). Ecological opportunity and sexual selection together predict adaptive radiation. Nature, 487, 366–369. [DOI] [PubMed] [Google Scholar]
- Wagner, C. E. , Harmon, L. J. , & Seehausen, O. (2014). Cichlid species‐area relationships are shaped by adaptive radiations that scale with area. Ecology Letters, 17, 583–592. [DOI] [PubMed] [Google Scholar]
- Willacker, J. J. , von Hippel, F. A. , Wilton, P. R. , & Walton, K. M. (2010). Classification of threespine stickleback along the benthic‐limnetic axis. Biological Journal of the Linnean Society, 101, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A. J. , Gislason, D. , Skúlason, S. , Snorrason, S. S. , Adams, C. E. , Alexander, G. , … Ferguson, M. M. (2004). Population genetic structure of Arctic charr, Salvelinus alpinus from northwest Europe on large and small spatial scales. Molecular Ecology, 13, 1129–1142. [DOI] [PubMed] [Google Scholar]
- Woods, P. J. , Skúlason, S. , Snorasson, S. S. , Kristjánsson, B. K. , Ingimarsson, F. , & Malmquist, H. J. (2013). Variability in the functional role of Arctic charr Salvelinus alpinus as it relates to lake ecosystem characteristics. Environmental Biology of Fishes, 96, 1361–1376. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


