Abstract
Juvenile nephronophthisis type 1 is caused by mutations of NPHP1, the gene encoding for nephrocystin. The function of nephrocystin is presently unknown, but the presence of a Src homology 3 domain and its recently described interaction with p130Cas suggest that nephrocystin is part of the focal adhesion signaling complex. We generated a nephrocystin-specific antiserum and analyzed the interaction of native nephrocystin with endogenous proteins. Immunoprecipitation of nephrocystin revealed that nephrocystin forms protein complexes with p130Cas, proline-rich tyrosine kinase 2 (Pyk2), and tensin, indicating that these proteins participate in a common signaling pathway. Expression of nephrocystin resulted in phosphorylation of Pyk2 on tyrosine 402 as well as activation of downstream mitogen-activated protein kinases, such as ERK1 and ERK2. Our findings suggest that nephrocystin helps to recruit Pyk2 to cell matrix adhesions, thereby initiating phosphorylation of Pyk2 and Pyk2-dependent signaling. A lack of functional nephrocystin may compromise Pyk2 signaling in a subset of renal epithelial cells.
Juvenile nephronophthisis (nephronophthisis type 1, NPH1) belongs to a group of genetically heterogeneous renal cystic diseases that follow an autosomal recessive mode of transmission (reviewed in ref. 1). Three different gene loci have been mapped, NPHP1 (juvenile form), NPHP2 (infantile form), and NPHP3 (adolescent form), that differ in the onset of end-stage renal disease. In addition, NPH1 can be associated with extrarenal manifestations (ocular motor apraxia, retinitis pigmentosa, coloboma of the optic nerve, cerebellar vermis aplasia, liver fibrosis, cone-shaped epiphyses) (reviewed in ref. 2). Recently, NPHP1, the gene mutated in juvenile nephronophthisis, has been identified (3, 4). NPH1 accounts for the majority of end-stage renal failure in children and young adults. The initial symptoms of the disease are relatively mild, consisting of polyuria and polydipsia due to a decreased capacity of the kidney to concentrate urine. Later, severe anemia, growth retardation, and development of end-stage renal failure at a median age of 13 years characterize the disease. The histological changes consist of tubular basement membrane disruption, interstitial cell infiltration, tubular atrophy, and cyst formation at the cortico-medullary junction (2, 5). The glomerula are not directly affected; however, a periglomerular fibrosis is evident at early stages of the disease.
NPHP1 encodes for nephrocystin, a protein with 732 aa residues and a predicted molecular mass of 83 kDa (3, 4). It contains an N-terminal coiled-coil structure and an Src homology 3 (SH3) domain that is flanked by two highly charged domains. Recently, the crk-associated substrate p130Cas was found to interact with the SH3 domain of nephrocystin in yeast two-hybrid screens using either p130Cas or nephrocystin as a bait (1, 6). Because p130Cas is an essential component of focal adhesions, it has been speculated that nephrocystin regulates the assembly of cell matrix adhesions. This hypothesis was further supported by the finding that nephrocystin appears to colocalize with E-cadherin at adherens junctions of cell–cell contacts in Madin–Darby canine kidney cells (6). Focal adhesions are formed by the clustering of β1- and αv-containing integrins through interaction with the extracellular matrix. Several intracellular proteins associate with focal adhesions, including tensin, talin, vinculin, α-actinin, paxillin, zyxin, vinexin, FAP52, nexilin, Src kinase, focal-adhesion kinase, protein kinase C, and calpain II (7, 8). Targeted disruption of certain components of the focal adhesion complex results in defects that closely resemble the characteristic features of human nephronophthisis. For example, mice lacking tensin develop multiple cysts, and the mice subsequently die from renal failure (9). In addition, the renal histology reveals the typical triad of NPH with tubular membrane disruption, tubular ectasia, and interstitial inflammation. Mice lacking α3β1, an integrin predominantly expressed in the podocyte, develop severe glomerular abnormalities but also display cystic changes of the proximal tubules (10). Finally, mice lacking GDIα, a GDP dissociation inhibitor of the small GTPase Rho, develop marked proteinuria as well as a degeneration of tubular epithelial cells (11). These findings indicate that abnormalities of the cell matrix adhesion complex may result in the development of tubular basement abnormalities in combination with renal cysts.
We demonstrate now that nephrocystin forms protein complexes, containing p130Cas, tensin, and proline-rich tyrosine kinase 2 (Pyk2). Nephrocystin triggers phosphorylation of tyrosine (402) of Pyk2 and subsequent activation of mitogen-activated protein kinases. Our findings suggest that nephrocystin is required for Pyk2-dependent signaling in certain tubular epithelial cells.
Materials and Methods
Plasmids.
FLAG-tagged versions of NPHP1 were generated by PCR and standard cloning techniques. Hemagglutinin (HA)-tagged and untagged versions of Pyk2 were a kind gift of I. Dikic (Ludwig Institute, Uppsala). R. Mulligan (Harvard Medical School, Boston) kindly provided the retroviral constructs pMD-G, pMD-gp, and pCMMP. To generate the retroviral gene transfer construct pCMMP.F.NPHP1.IRES.gfp, a FLAG-tag and a multiple cloning site were inserted into the vector pCMMP, followed by an internal ribosome entry site and green fluorescent protein (GFP). The control vector contains the internal ribosome entry site followed by GFP.
Cell Culture and Retrovirus Production.
Inner-medullary collecting duct (IMCD) cells were grown in DMEM-F12 supplemented with 10% FCS. The retroviral vector was produced by cotransfection of HEK 293T cells with three plasmids by using the calcium phosphate method. Briefly, 2.5 μg of pMD-G, 7.5 μg of pMD-gp, and 10 μg of the retroviral transfer vector were cotransfected into HEK 293T. The supernatant was harvested, centrifuged to remove cellular debris, and filtered. Typically, more than 95% of cells expressed the transgene after three repetitive infections.
Generation and Purification of a Nephrocystin-Specific Antiserum.
A recombinant, gel-purified nephrocystin fragment (amino acids 1–209) fused to the maltose-binding protein (MBP) [MBP-NPHP1 (1)] was used to immunize rabbits (Cocalico Biologicals, Reamstown, PA), following a standard immunization protocol. For affinity purification of the rabbit antiserum, the recombinant MBP-NPHP1 (1) was coupled to AminoLink Plus (Pierce); immobilized nephrocystin-specific antiserum was eluted from the column with 0.1 M glycine-HCl (pH 3.0) and dialyzed against PBS. The two control proteins, glutathione S-transferase (GST).nephrin and GST.INV, were generated by fusing GST with amino acids 1087–1241 of nephrin and with amino acids 561–716 of inversin, respectively. All recombinant proteins were expressed in Escherichia coli MC1061, following standard protocols.
Western Blot Analysis.
Western blot analysis was performed as described (12). Phosphorylation of tyrosine 402 of Pyk2 was monitored with a phosphospecific antiserum (Quality Control Biochemicals, Hopkington, MA), whereas overall tyrosine phosphorylation was visualized with the monoclonal antibody 4G10 (Upstate Biotechnology, Lake Placid, NY). All other antibodies were from BD-Transduction (Heidelberg, Germany) (anti-Pyk2, anti-p130Cas, anti-tensin), Sigma (anti-FLAG M2), and Roche Molecular Biochemicals (anti-HA). Equal protein loading was confirmed by amidoblack staining of the membranes.
Subcellular Fractionation.
IMCD cells, retrovirally transduced to express nephrocystin, were harvested and lysed at 4°C in a glass/glass homogenizer in 1 ml of 250 mM sucrose/1 mM EDTA/20 mM Tris, pH 7.5/44 μg/ml phenylmethylsulfonyl fluoride and protease inhibitors. Nuclei were removed by centrifugation at 1,000 × g for 10 min at 4°C. The postnuclear supernatant was centrifuged at 100,000 × g for 30 min at 4°C. The supernatant (S100 soluble fraction) was collected, and the pellet (P100 membrane fraction) was washed once in homogenization buffer and then resuspended in lysis buffer containing 1% Triton X-100 and 0.2% SDS. Protein concentration was determined, and equal amounts of protein were separated by 10% SDS/PAGE.
Coimmunoprecipitations.
Thirty embryonic kidneys and testes were harvested from pregnant mice at embryonic day 13.5 (Charles River Breeding Laboratories), pooled, and homogenized in 1 ml of lysis buffer (20 mM Tris-HCl, pH 7.5/0.5% Triton X-100/25 mM NaF/12.5 mM Na4P2O7/0.1 mM EDTA/50 mM NaCl/2 mM Na3VO4 and protease inhibitors). After centrifugation to remove cellular debris, the supernatant was subjected to an ultracentrifugation (100,000 × g) for 30 min, followed by extensive preclearing with protein G Sepharose. Immunoprecipitation with control antibody or anti-nephrocystin antiserum was performed as described (12).
In Vitro Protein Interaction.
Purified recombinant protein (1 μg of GST.Pyk2-PR, representing the proline-rich region of Pyk2 or GST alone) was immobilized on glutathione Sepharose resin and incubated with purified recombinant MBP.NPHP1 (1) for 90 min in 450 μl of binding buffer containing 50 mM potassium phosphate, pH 7.5/150 mM KCl/1 mM MgCl2/10% (vol/vol) glycerol/1% Triton X-100 and protease inhibitors. The precipitate was washed and separated on a 10% SDS gel. Bound MBP.NPHP1 (1) was detected by immunoblotting.
Results
Nephrocystin Interacts with Pyk2 in Mouse Embryonic Kidney.
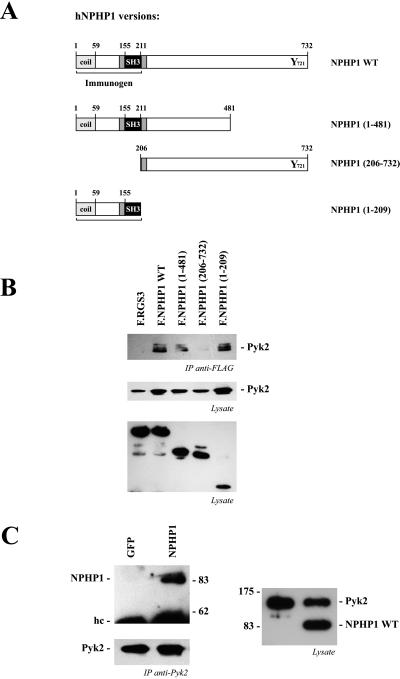
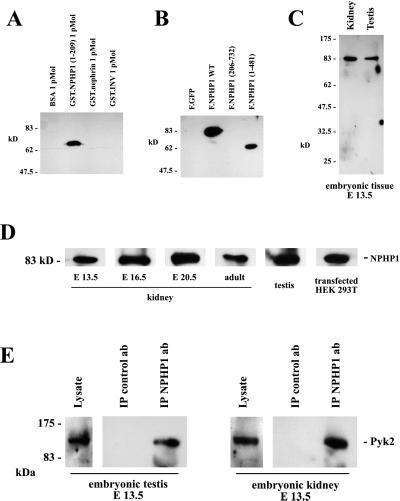
Nephrocystin has been reported to interact with the focal adhesion protein p130Cas (6). Because the targeted disruption of several focal adhesion proteins results in tubular basement abnormalities and renal cyst formation (reviewed in ref. 1), we tested whether other focal adhesion molecules interact with nephrocystin. Pyk2 coimmunoprecipitated with Flag-tagged nephrocystin and nephrocystin deletion constructs (Fig. 1A) that contained the SH3 domain (amino acids 155–211), whereas there was no interaction of Pyk2 with a nephrocystin deletion that lacked the first 205 aa (Fig. 1B). The reciprocal immunoprecipitation of Pyk2 and detection of nephrocystin in the precipitates (Fig. 1C) provided further evidence for the interaction between Pyk2 and nephrocystin. To test whether this interaction occurs in vivo, we examined the interaction of endogenous proteins in embryonic tissues. Using an MBP-nephrocystin fusion protein that contained the first 209 aa of human nephrocystin, we generated a rabbit antiserum that specifically recognized recombinant GST-nephrocystin, but not two other control GST-fusion proteins (Fig. 2A). A GST-nephrocystin agarose column was used to affinity-purify the nephrocystin-specific antiserum. Western blotting demonstrated that this antiserum detects a single protein in HEK 293T cells expressing either full-length nephrocystin or the truncated version of nephrocystin F.NPHP1(1–481), containing the first 481 aa of nephrocystin (Fig. 2B). In contrast, the nephrocystin deletion construct F.NPHP1(206–732), lacking the first 205 aa, was not recognized by the antiserum. Human and mouse nephrocystin are 83% identical (13). To test whether mouse nephrocystin can be detected in embryonic tissues, mouse embryonic kidneys and testes were lysed, and the solubilized proteins were separated on a 10% SDS/PAGE. Western blot analysis revealed that the antiserum detects a specific band at the predicted size in mouse tissues (Fig. 2 C and D). Using this antiserum, we examined whether endogenous nephrocystin interacts with endogenous Pyk2. Embryonic mouse kidneys and testes were lysed in lysis buffer and spun at 100,000 × g to remove insoluble material. Nephrocystin was precipitated from the supernatant, using the affinity-purified antiserum in combination with protein A Sepharose. As demonstrated in Fig. 2E, Pyk2 interacts with nephrocystin in the embryonic kidney as well as the embryonic testis. In contrast to Pyk2, only a weak interaction was detectable between nephrocystin and the focal adhesion kinase FAK in HEK 293T cells, overexpressing both proteins; no interaction was observed between these two proteins in native tissues (data not shown).
Figure 1.
Nephrocystin interacts with Pyk2. (A) Several Flag-tagged nephrocystin truncations were generated to analyze the interaction with Pyk2. Nephrocystin contains several putative protein–protein interaction motifs, including an amino-terminal coiled-coil domain (coil) and an SH3 domain. (B) Nephrocystin precipitates Pyk2. HA-tagged Pyk2 and Flag-tagged nephrocystin constructs were expressed in HEK 293T cells and precipitated with a Flag-specific antibody. Western blot analysis was performed with an HA-specific antiserum (Top and Middle). Pyk2 coprecipitates with nephrocystin but not with the control protein RGS3 (Top). The interaction requires the N-terminal region of nephrocystin, containing the SH3 domain. (Middle) Expression levels of HA.Pyk2 are shown. (Bottom) Expression levels of Flag-tagged RGS3 and nephrocystin constructs in cell lysates are shown. (C) Pyk2 precipitates nephrocystin but not the control protein GFP (Left Upper); the Pyk2 precipitates are shown (Left Lower). (Right) The expressions of Flag-tagged nephrocystin and HA-tagged Pyk2 in the cellular lysates are shown.
Figure 2.
Interaction between endogenous nephrocystin and Pyk2. (A and B) To generate a nephrocystin-specific antiserum, rabbits were immunized with a recombinant MBP fusion protein, containing the amino-terminal 209 aa of nephrocystin. Affinity-purified antiserum specifically recognized recombinant nephrocystin fused to GST and nephrocystin expressed in HEK 293T cells, but failed to bind recombinant control proteins or a nephrocystin version lacking the region used for immunization. (C and D) The antiserum recognizes mouse nephrocystin in endogenous tissues. To test whether mouse nephrocystin can be detected in embryonic tissues, mouse embryonic kidneys and testes were lysed at different developmental stages, and the solubilized proteins were separated on a 10% SDS/PAGE. Western blot analysis revealed that the antiserum raised against human nephrocystin detects mouse nephrocystin. (E) Nephrocystin interacts with Pyk2 in embryonic tissues. The nephrocystin antiserum was used to examine the endogenous interaction between Pyk2 and nephrocystin in the embryonic mouse kidney and testis. Embryonic kidneys (Right) and embryonic testes (Left) were collected and homogenized. The lysates were immunoprecipitated with a control antiserum or the nephrocystin-specific antiserum. Western blot analysis was performed with a Pyk2-specific antiserum.
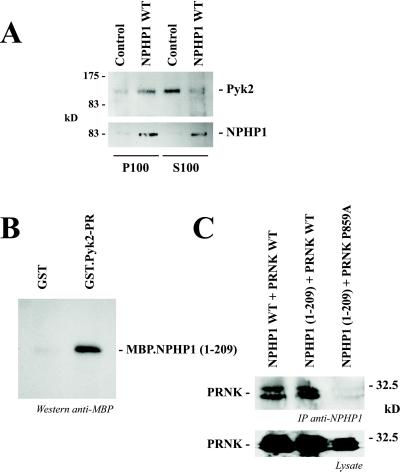
The interaction with nephrocystin affected the subcellular localization of Pyk2. IMCD cells, a mouse cell line derived from IMCDs, express endogenous Pyk2 but lack nephrocystin. We generated a retrovirus to express nephrocystin in these cells. Because more than 90% of IMCD cells expressed nephrocystin after three subsequent infections, experiments were performed without further clonal selection. As shown in Fig. 3A, the expression of nephrocystin in IMCD cells resulted in a striking increase of membrane-associated Pyk2 (P100 fraction) associated with a simultaneous decrease of cytosolic Pyk2 (S100 fraction).
Figure 3.
Subcellular localization of Pyk2 and direct interaction between nephrocystin and the proline-rich (PR) region of Pyk2. (A) Nephrocystin modulates the subcellular localization of Pyk2. The tubular epithelial cell line IMCD was infected with a retrovirus, directing the expression of nephrocystin, or a control retrovirus. Nephrocystin increased the membrane-associated fraction (P100) and decreased the cytoplasmic fraction (S100) of Pyk2. (B) GST and GST.Pyk2-PR were immobilized on glutathione-Sepharose beads and incubated with MBP.NPHP1 (1). NPHP1 binds to GST.Pyk2-PR but not to GST. (C) Coimmunoprecipitation of PRNK, a naturally occurring splice variant of Pyk2, with full-length nephrocystin (NPHP1 WT) or the C-terminally truncated version [NPHP1 (1)]. (Upper) Mutation of the proline residue 859 in Pyk2 abrogates the interaction with nephrocystin. (Lower) Expression of wild-type and mutated PRNK is shown.
Because nephrocystin binds via its SH3 domain to p130Cas (6), whereas the SH3 domain of p130Cas can interact with Pyk2 (14), it is conceivable that the interaction between nephrocystin and Pyk2 is mediated by p130Cas. To demonstrate that the SH3 domain of nephrocystin directly interacts with Pyk2, recombinant MBP-nephrocystin 1–209 was immobilized to amylose beads and incubated with a GST-fusion protein that contained the proline-rich domain of Pyk2 (amino acids 669–949). As demonstrated in Fig. 3B, GST-nephrocystin retained Pyk2, confirming that the interaction between nephrocystin and Pyk2 is direct and does not require an intermediary such as p130Cas. The Pyk2-related non-kinase (PRNK) is a naturally occurring splice variant of Pyk2 representing only a C-terminal region of Pyk2 (15). Whereas HA-tagged PRNK interacted with either wild-type nephrocystin or the SH3-containing nephrocystin truncation (amino acids 1–209), the proline 859-to-alanine mutation of PRNK completely abrogated this interaction, indicating that this proline is critical for the interaction between nephrocystin and Pyk2 (Fig. 3C).
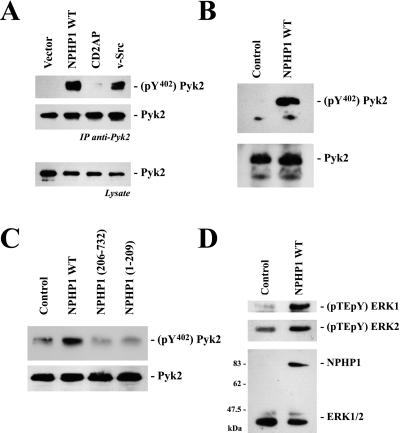
Nephrocystin Triggers Phosphorylation of Tyrosine 402 of Pyk2.
To test whether the presence of nephrocystin alters the activity of Pyk2, an antiserum was used that specifically recognizes the tyrosine (402)-phosphorylated form of Pyk2. As demonstrated in Fig. 4A, expression of either nephrocystin or v-src triggered the phosphorylation of tyrosine (402) of Pyk2. In contrast, CD2AP, another SH3-containing adapter molecule, or vector control had no effect on the phosphorylation status of tyrosine (402) of Pyk2. In several independent experiments (n > 3), the expression of nephrocystin triggered phosphorylation of tyrosine 402 of Pyk2 that was clearly detectable using the pY (402)-specific antiserum (Fig. 4B). No tyrosine (402) phosphorylation of Pyk2 was detectable in IMCD cells infected with a retrovirus that directed the expression of the control protein, GFP (Fig. 4B). Interestingly, full-length nephrocystin is required to trigger phosphorylation of tyrosine (402) of Pyk2. As demonstrated in Fig. 4C, truncation of either the N-terminal or the C-terminal domain of nephrocystin abrogates the capacity of nephrocystin to trigger phosphorylation of Pyk2. Phosphorylation of tyrosine (402) of Pyk2 triggers the recruitment of Src-family kinases and interaction with other adapter and effector molecules (reviewed in refs. 16 and 17). We found an increased activity of the mitogen-activated protein kinases ERK1 and ERK2 in IMCD cells that expressed nephrocystin, but not in IMCD cells that expressed the control protein GFP (Fig. 4D).
Figure 4.
Nephrocystin triggers phosphorylation of tyrosine 402 of Pyk2. (A) HEK 293T cells were transfected with HA-tagged Pyk2 in combination with vector control, nephrocystin, the SH3 domain-containing adapter protein CD2AP, or the positive control v-Src. HA.Pyk2 was immunoprecipitated from serum-starved cells with anti-HA antibody; precipitates were probed with an antiserum detecting phosphotyrosine 402 of Pyk2 (Top) or with an antiserum revealing the total amount of Pyk2 (Middle). (Bottom) The expression of HA.Pyk2 in the lysates. (B) Pyk2 is phosphorylated on tyrosine 402 in nephrocystin-expressing IMCD cells. IMCD cells were infected with either a control or a nephrocystin-expressing retrovirus. Nephrocystin induces tyrosine 402 phosphorylation of endogenous Pyk2 in IMCD cells (Upper) but does not influence the expression levels of Pyk2 (Lower). (C) Full-length nephrocystin is required to trigger phosphorylation of tyrosine 402 of Pyk2. Retroviral vectors, expressing nephrocystin truncations, were constructed to infect IMCD cells. Both the amino-terminal deletion [NPHP1 (206)] and the carboxyl-terminal deletion [NPHP1 (1)] of nephrocystin failed to mediate tyrosine 402 phosphorylation of Pyk2 (Upper). (Lower) The levels of Pyk2 expression are shown. (D) Nephrocystin activates ERK1/2. Dually phosphorylated ERK1 and ERK2 was visualized in IMCD cells, transduced with control vector or a retroviral vector encoding for nephrocystin, using a phosphospecific antiserum (Upper). The blots were reprobed with an antiserum detecting total amounts of ERK1 and ERK2 and with the nephrocystin-specific antiserum.
P130Cas and Tensin Are Present in Nephrocystin-Containing Immunoprecipitates.
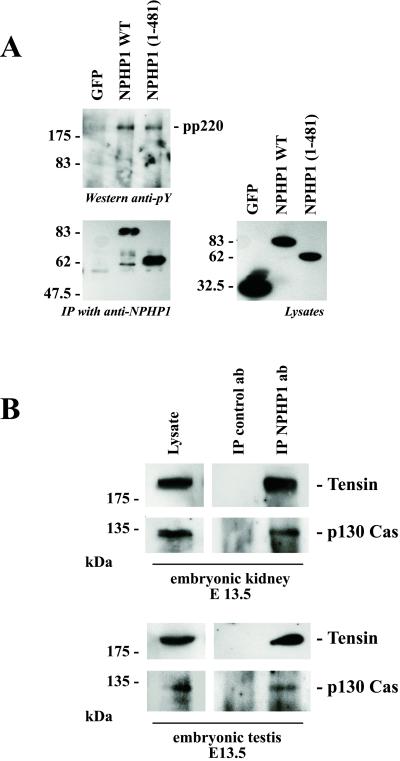
Recently, a direct interaction was identified between nephrocystin and p130Cas by using the yeast two-hybrid system (6). This finding prompted us to examine whether nephrocystin interacts with p130Cas in endogenous tissues. As shown in Fig. 5, p130Cas was present in the immune complexes immobilized by anti-nephrocystin antiserum in both embryonic kidney and embryonic testis. In HEK 293T cells, wild-type nephrocystin as well as the N-terminal nephrocystin construct (amino acids 1–481) precipitated a tyrosine-phosphorylated protein of ≈220 kDa that was not detectable after precipitation of the C-terminal nephrocystin construct (Fig. 5). Because targeted disruption of tensin, a 220-kDa phosphoprotein, results in a phenotype that very closely resembles human nephronophthisis (9), we tested whether tensin was associated with endogenous nephrocystin in mouse embryonic kidneys. Using a tensin-specific antiserum, tensin was clearly detectable in the nephrocystin-containing immunoprecipitates. Further studies will need to clarify whether coimmunoprecipitation of tensin is mediated by a direct interaction with nephrocystin.
Figure 5.
Nephrocystin associates with p130Cas and tensin. (A) To identify nephrocystin-interacting proteins, HEK 293T cells were transfected with the control protein GFP, nephrocystin, or the nephrocystin truncation NPHP1 (1). After immunoprecipitation of nephrocystin, immunoprecipitates were separated on a 10% SDS/PAGE and stained with the monoclonal antiphosphotyrosine antibody 4G10 (Left Upper), or anti-Flag antibody (Left Lower). A tyrosine-phosphorylated protein with an approximate molecular mass of 220 kDa (pp220) was detected in the nephrocystin precipitates. Expression levels of Flag-tagged GFP and nephrocystin truncations in the lysates are demonstrated (Right). HEK 293T cells lack endogenous Pyk2, and consequently Pyk2 was not detectable in the 4G10-labeled precipitates. (B) Coprecipitation of tensin and p130Cas with native nephrocystin from embryonic tissues. Embryonic kidneys (Upper) and embryonic testes (Lower) were collected and homogenized. The lysates were immunoprecipitated with a control or nephrocystin-specific antiserum and separated by 10% SDS/PAGE. Tensin and p130Cas were detected in the nephrocystin-containing precipitates using tensin or p130Cas-specific antibodies.
Discussion
Homozygous mutations of nephrocystin cause nephronophthisis type I (reviewed in ref. 1). This disease is characterized by the development of medullary renal cysts and renal failure during adolescence, heralded by polydipsia and polyuria. The histological changes are characterized by thickening, splintering, and disruption of the tubular basement membrane in combination with progressive atrophy of the tubular epithelial cells (2, 5, 18). Because the basement membrane abnormalities appear to occur before cyst formation, it has been postulated that the alteration of the tubular basement membrane is the principle defect causing the phenotypic changes observed in nephronophthisis. How nephrocystin affects the integrity of tubular basement membranes is currently unknown. The presence of a coiled-coil structure in combination with an SH3 domain suggests that this molecule functions as an adapter protein. The recently described interaction of nephrocystin with the focal adhesion protein p130Cas and its colocalization with focal adhesions in polarized epithelial cells indicate that nephrocystin plays a role in focal adhesion signaling of renal tubular epithelial cells (6). We demonstrate now that nephrocystin interacts directly with Pyk2 and forms protein complexes with p130Cas, Pyk2, and tensin in embryonic kidneys and testis.
The nonreceptor tyrosine kinase Pyk2 is activated by stimuli that raise intracellular Ca2+ levels or engage β1 integrins. Src-family kinases interact with Pyk2 following its autophosphorylation on tyrosine 402 and mediate activation of mitogen-activated protein kinases (reviewed in refs. 16 and 17). Pyk2 shares with the focal adhesion kinase FAK the dependence of actin filament integrity for its function as well as the ability to interact with p130Cas. In many tissues, Pyk2 is not primarily localized to focal adhesions but appears to predominantly reside in the cytoplasm (19). Because nephrocystin is present in the membrane as well as the cytosolic compartment (Fig. 3A), it is conceivable that nephrocystin serves as a shuttle protein that mediates translocation of Pyk2 from the cytoplasm to cell matrix adhesions, where it is activated in response to extracellular signals. Pyk2, phosphorylated on tyrosine 402, can recruit Src-kinase family members that activate mitogen-activated protein kinases. Indeed, we found elevated levels of activated ERK1 and ERK2 in IMCD cells expressing nephrocystin (Fig. 4D).
P130Cas is a Pyk2 substrate, and tyrosine phosphorylation of p130Cas by Pyk2 promotes binding of Crk (14, 20), which in turn can stimulate cell migration in a Rac-dependent manner (21). Interestingly, Pyk2 does not effectively reconstitute cellular migration in response to fibronectin in fibroblasts that lack focal adhesion kinase (22). The inability of Pyk2 to effectively stimulate migration of certain cell types is not related to differences in signaling between focal adhesion kinase and Pyk2, but seems to result from insufficient recruitment of Pyk2 to focal contacts (reviewed in ref. 16). We propose that nephrocystin facilitates the recruitment of Pyk2 to specialized cell matrix adhesions in response to extracellular signals. Interestingly, levels of α5β1 integrin receptors are increased on tubular epithelial cells of patients with nephronophthisis type I (23). Typically, α5β1 integrins translocate away from focal contacts, reorganize the fibronectin molecules of the extracellular matrix, and form fibrillary adhesions along bundles of actin filaments (24, 25). These fibrillar adhesions contain high levels of tensin and promote cellular mobility but contain low levels of paxillin, vinculin, and tyrosine-phosphorylated proteins. Tensin is a critical component of fibrillary adhesions, and a dominant-negative inhibitor of tensin blocks the formation of this type of extracellular matrix contact (25). It is likely that the formation of fibrillary adhesions is altered in tensin-deficient mice and perhaps contributes to the development of cystic renal disease in these animals (9). Because tensin and nephrocystin seem to participate in a common signaling pathway, it is tempting to speculate that tubular epithelial cells may lose their ability to reorganize fibronectin and to form fibrillar adhesions in the absence of either tensin or nephrocystin. Taken together, our findings indicate that nephrocystin, p130Cas, Pyk2, and tensin participate in a common signaling pathway that may be required for specific types of cell matrix contacts by a subset of tubular epithelial cells.
Acknowledgments
We thank Dr. I. Dikic (Ludwig Institute for Cancer Research, Uppsala, Sweden) for several cDNAs and Dr. A. Blaukat and members of the Walz laboratory for helpful comments. We gratefully acknowledge the expert technical assistance of Christina Engel and Birgit Schilling. This work was supported by Deutsche Forschungsgemeinschaft Grant WA 597 and by a grant from the Thyssen-Stiftung.
Abbreviations
- NPH
nephronophthisis
- Pyk2
proline-rich tyrosine kinase 2
- PRNK
Pyk2-related non-kinase
- p130Cas
p130 crk-associated substrate
- SH3
Src homology 3
- NPHP1
nephronophthisis type 1
- HA
hemagglutinin
- GFP
green fluorescent protein
- MBP
maltose-binding protein
- GST
glutathione S-transferase
- IMCD
inner-medullary collecting duct
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hildebrandt F, Otto E. J Am Soc Nephrol. 2000;11:1753–1761. doi: 10.1681/ASN.V1191753. [DOI] [PubMed] [Google Scholar]
- 2.Waldherr R, Lennert T, Weber H P, Fodisch H J, Scharer K. Virchows Arch Pathol Anat Physiol Klin Med. 1982;394:235–254. doi: 10.1007/BF00430668. [DOI] [PubMed] [Google Scholar]
- 3.Hildebrandt F, Otto E, Rensing C, Nothwang H G, Vollmer M, Adolphs J, Hanusch H, Brandis M. Nat Genet. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 4.Saunier S, Calado J, Heilig R, Silbermann F, Benessy F, Morin G, Konrad M, Broyer M, Gubler M C, Weissenbach J, Antignac C. Hum Mol Genet. 1997;6:2317–2323. doi: 10.1093/hmg/6.13.2317. [DOI] [PubMed] [Google Scholar]
- 5.Sherman F E, Studnicki F M, Fetterman G. Am J Clin Pathol. 1971;55:391–400. doi: 10.1093/ajcp/55.4.391. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson J C, Dempsey P J, Reddy S, Bouton A H, Coffey R J, Hanks S K. Exp Cell Res. 2000;256:168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- 7.Brugge J S. Nat Genet. 1998;19:309–311. doi: 10.1038/1189. [DOI] [PubMed] [Google Scholar]
- 8.Giancotti F G, Ruoslahti E. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 9.Lo S H, Yu Q C, Degenstein L, Chen L B, Fuchs E. J Cell Biol. 1997;136:1349–1361. doi: 10.1083/jcb.136.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreidberg J A, Donovan M J, Goldstein S L, Rennke H, Shepherd K, Jones R C, Jaenisch R. Development (Cambridge, UK) 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 11.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, et al. Oncogene. 1999;18:5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 12.Benzing T, Yaffe M B, Arnould T, Sellin L, Schermer B, Schilling B, Schreiber R, Kunzelmann K, Leparc G G, Kim E, Walz G. J Biol Chem. 2000;275:28167–28172. doi: 10.1074/jbc.M002905200. [DOI] [PubMed] [Google Scholar]
- 13.Otto E, Kispert A, Schatzle S, Lescher B, Rensing C, Hildebrandt F. J Am Soc Nephrol. 2000;11:270–282. doi: 10.1681/ASN.V112270. [DOI] [PubMed] [Google Scholar]
- 14.Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 15.Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth B U, Dikic I. Nat Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- 16.Schlaepfer D D, Hauck C R, Sieg D J. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 17.Avraham H, Park S Y, Schinkmann K, Avraham S. Cell Signalling. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara K, Suzuki K, Lin Y W, Yamamoto T, Ohta S. Acta Paediatr Jpn. 1991;33:482–487. doi: 10.1111/j.1442-200x.1991.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 19.Schaller M D, Sasaki T. J Biol Chem. 1997;272:25319–25325. doi: 10.1074/jbc.272.40.25319. [DOI] [PubMed] [Google Scholar]
- 20.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemke R L, Leng J, Molander R, Brooks P C, Vuori K, Cheresh D A. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueki K, Mimura T, Nakamoto T, Sasaki T, Aizawa S, Hirai H, Yano S, Naruse T, Nojima Y. FEBS Lett. 1998;432:197–201. doi: 10.1016/s0014-5793(98)00862-x. [DOI] [PubMed] [Google Scholar]
- 23.Rahilly M A, Fleming S. Histopathology. 1995;26:345–349. doi: 10.1111/j.1365-2559.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 24.Katz B Z, Zamir E, Bershadsky A, Kam Z, Yamada K M, Geiger B. Mol Biol Cell. 2000;11:1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankov R, Cukierman E, Katz B Z, Matsumoto K, Lin D C, Lin S, Hahn C, Yamada K M. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]