Abstract
Purpose
To describe the development of a conceptual model to guide research focused on lung cancer screening participation from the perspective of the individual in the decision-making process.
Methods
Based on a comprehensive review of empirical and theoretical literature, a conceptual model was developed linking key psychological variables (stigma, medical mistrust, fatalism, worry, and fear) to the health belief model and precaution adoption process model.
Results
Proposed model concepts have been examined in prior research of either lung or other cancer screening behavior. To date, a few studies have explored a limited number of variables that influence screening behavior in lung cancer specifically. Therefore, relationships among concepts in the model have been proposed and future research directions presented.
Conclusion
This proposed model is an initial step to support theoretically based research. As lung cancer screening becomes more widely implemented, it is critical to theoretically guide research to understand variables that may be associated with lung cancer screening participation. Findings from future research guided by the proposed conceptual model can be used to refine the model and inform tailored intervention development.
Keywords: conceptual model, lung cancer screening, individual participation, patients
Lung cancer screening is a relatively new recommendation, and although numerous conceptual models have been developed to explain other types of cancer screening (i.e., breast, colorectal, prostate), the population targeted for lung cancer screening is unique. Individuals eligible for lung cancer screening are long-term tobacco smokers, and smokers are a population different from those targeted for other types of cancer screening. Smokers experience stigma, battle an addiction to nicotine, and perceive blame from others related to the perceived self-infliction of tobacco-related diseases secondary to their lifestyle choice, and in turn, individual health beliefs related to cancer screening may be influenced by an individuals’ smoking status. Based on our preliminary qualitative work, variables such as health beliefs (perceived risk, perceived benefits, perceived barriers, and self-efficacy) as well as perceived stigma, medical mistrust, cancer fatalism, fear, and worry seem to be uniquely relevant in lung cancer screening (Carter-Harris, Ceppa, Hanna, & Rawl, 2015).
For context, annual lung cancer screening with low-dose computed tomography (LDCT) is a recent recommendation approved by the U.S. Preventive Services Task Force (USPSTF) for long-term current and former smokers (Wender et al., 2013). Lung cancer kills more people worldwide than breast, colorectal, pancreatic, and prostate cancers combined each year (World Health Organization, 2012). Most die because they are diagnosed at an advanced stage with limited treatment options and, until recently, an effective screening test to identify lung cancer at an earlier stage did not exist (Boiselle, 2013). However, the U.S. National Lung Screening Trial of more than 53,000 long-term smokers found a significant benefit for annual chest LDCT versus chest radiography, with a 20% relative reduction in lung cancer—related mortality (American Cancer Society, 2015).
In response, the USPSTF issued national guidelines recommending annual chest LDCT for long-term smokers (USPSTF, 2013) and a Grade B recommendation reflecting their conclusion that sufficient evidence was available supporting a moderate to substantial benefit of annual chest LDCT with high certainty for long-term current and former smokers (USPSTF, 2013). Lung cancer screening is therefore recommended for individuals age 55–77 years with a minimum 30 pack-year tobacco smoking history who currently smoke or have quit within the past 15 years (USPSTF, 2014). Pack-year is defined as the number of packs of cigarettes smoked daily multiplied by the number of years smoked total.
Lung cancer screening is increasingly available in the United States as well as other countries, and U.S. health care systems are rapidly increasing their number of screening programs (Lung Cancer Alliance, 2015). In addition, although lung cancer screening has benefits, there are associated harms. Therefore, a shared decision-making process between an individual and his or her health care provider is essential to help the individual weigh the benefits against the harms specific to their circumstance to result in an informed screening decision. Shared decision-making has been defined as a collaborative process occurring between an individual and their health care provider where patients are supported to consider their options informed by consideration of the best available scientific evidence and the individual’s values and preferences (Elwyn et al., 2012). Lung cancer screening is the first, and only, cancer screening modality to date in the United States that requires documentation of shared decision-making for reimbursement and offers a fertile ground on which to conceptually frame the individuals’ perspective of the decision to screen, or not, for lung cancer.
There is a gap in extant knowledge as to why screening-eligible smokers decide to, or not to, screen for lung cancer. Lung cancer screening participation is influenced at multiple levels, and although there are numerous models in other types of cancer screening, conceptual models not tested specifically in lung cancer may not have the same findings. Linking the uniquely important psychological variables (i.e., stigma, mistrust, fatalism, fear, and worry) with traditional health belief model (HBM) constructs and other important variables has the potential to offer a more complete understanding of lung cancer screening behavior: knowledge that is essential for tailoring future interventions with this high-risk population.
A strong body of literature supports models of health promotion and risk reduction frameworks that have been successfully tested over time and guided research on preventive screening behaviors broadly (Bandura, 1998; Flynn, Betancourt, & Ormseth, 2011; Pender, 1975). However, lung cancer screening guidelines are based on age and history of long-term tobacco use. As mentioned previously, smokers are a unique population different from those targeted for other types of cancer screening because of a lifestyle choice and there may be factors that influence both the shared decision-making process as well as the ultimate outcome, lung cancer screening participation, that necessitate a new model to guide research in this area.
The current state of the science in this specific cancer screening area is defined by descriptive, qualitative research (Byrne, Weissfeld, & Roberts, 2008; Carter-Harris et al., 2015; Jonnalagadda et al., 2012; Patel et al., 2012), which has given important insight into the patients’ perspective related to lung cancer screening participation. However, as lung cancer screening programs continue to become more widely implemented, it is essential to have a conceptual model guiding research to understand important variables that influence the decision to screen and subsequent screening behavior. Therefore, the purpose of this article is to describe the development of such a conceptual model from the perspective of the individual in the decision-making process. We acknowledge that lung cancer screening participation also involves provider and health care system factors that influence this decision-making process. However, developing effective patient-tailored interventions requires an initial framework from the perspective of the individual making this important screening participation decision.
DEVELOPMENT OF THE CONCEPTUAL MODEL
Drawing from theoretical and empirical literature, the conceptual model for lung cancer screening participation was developed initially by the authors to frame our understanding of the decision to screen for lung cancer in long-term current and former smokers. Because lung cancer screening is a recent recommendation, there is a dearth of literature specific to why long-term smokers screen versus not for lung cancer. Studies exploring attitudes and beliefs about lung cancer screening, including our own, were used to support what variables were included in this initial model (Byrne et al., 2008; Carter-Harris et al., 2015; Jonnalagadda et al., 2012; Patel et al., 2012). Because this initial qualitative research in lung cancer screening specifically did not include all potential influential variables given the nascent state of the science, the authors turned to the literature in other types of cancer screening to consider variables for inclusion that may have similar associations in lung cancer screening. In addition, the indirect relationships presented are theoretically and empirically supported by literature in other types of cancer screening such as colorectal and breast. It is possible when all relationships in this initial model are fully tested, the model will change as a result of refinement, increased understanding of direct versus indirect relationships, and potential for a variable to not be applicable in lung cancer screening specifically. The remainder of this article presents the variables included in this initial model as a review of the literature to support why each variable was included.
THEORETICAL FOUNDATIONS
The conceptual model for lung cancer screening participation conceptualizes screening participation from the perspective of the individual and depicts psychological variables as key factors in lung cancer screening. In addition, the psychological variables are linked to (a) the traditional HBM constructs that have predicted participation in screening for other cancers (Carpenter, 2010); (b) important components of the shared decision-making process (Sheridan, Harris, & Woolf, 2004); and (c) the stage theory, precaution adoption process model (PAPM; Weinstein, Sandman, & Blalock, 2008). Linking uniquely important psychological variables with traditional HBM constructs, the shared decision-making process, and the PAPM has the potential to offer a more complete understanding of lung cancer screening behavior. The HBM is an established model used to explain many other types of health behavior including other types of cancer screening (Menon et al., 2007; Rawl et al., 2005), and it is appropriate in the context of lung cancer screening. The shared decision-making process is an essential component of the decision to screen, or not, for lung cancer (USPSTF, 2014), and the PAPM has been suggested in other types of cancer screening as a useful model to assess an individual’s readiness to undergo screening because it accounts for those individuals who decide not to screen (Costanza et al., 2005). Our conceptual model was developed a priori, drawing on theoretical and empirical cancer screening literature in lung and other cancers to guide model development. Variables shown to be associated qualitatively or quantitatively with lung (Carter-Harris et al., 2015; Jonnalagadda et al., 2012; Patel et al., 2012; Tanner, Egede, Shamblin, Gebregziabher, & Silvestri, 2013) or other cancer screening behaviors (Menon et al., 2007) have been included.
The decision to participate in lung cancer screening is a complex process. We propose that it is influenced by psychological variables, demographic and health status characteristics, cognitive variables, receiving a health care provider recommendation, social and environmental variables, and lung cancer screening health beliefs. We further acknowledge the importance of the shared decision-making process between an individual and their health care provider in lung cancer screening.
LITERATURE REVIEW AND EMPIRICAL AND/OR THEORETICAL SUPPORT FOR VARIABLES INCLUDED IN THE CONCEPTUAL MODEL
Research exploring health beliefs in lung cancer screening is still nascent, with early qualitative work exploring attitudes and beliefs from the perspective of long-term smokers (Carter-Harris et al., 2015; Delmerico, Hyland, Celestino, Reid, & Cummings, 2014; Jonnalagadda et al., 2012; Patel et al., 2012; Silvestri, Nietert, Zoller, Carter, & Bradford, 2007; Tanner et al., 2013) and health care providers (Klabunde et al., 2010). These findings, in addition to foundational theoretical literature, were used to develop the proposed conceptual model of lung cancer screening participation (Delmerico et al., 2014; Jonnalagadda et al., 2012; Klabunde et al., 2010; Patel et al., 2012; Silvestri et al., 2007; Tanner et al., 2013). Although most studies specific to lung cancer screening have been atheoretical (Delmerico et al., 2014; Patel et al., 2012; Silvestri et al., 2007; Tanner et al., 2013), one study used self-regulation theory (Jonnalagadda et al., 2012) and our initial research used the HBM (Carter-Harris et al., 2015) to explore lung cancer screening health beliefs. Four other studies examined the HBM constructs of perceived risk and perceived barriers without formally making the theoretical link (Delmerico et al., 2014; Patel et al., 2012; Silvestri et al., 2007; Tanner et al., 2013).
Little is known about why screening-eligible smokers decide to or not to screen for lung cancer. A comprehensive review of the literature was conducted to inform development of the conceptual model (Figure 1). The rationales for including each variable in the model are presented in the following text. Because lung cancer screening is new, no studies have examined stage of adoption for lung cancer screening participation. Rather, most research in this area has focused on factors associated with interest in screening or intent to screen rather than the decision-making process and actual screening behavior (Hahn, Rayens, Hopenhayn, & Christian, 2006; Tanner et al., 2013). Because lung cancer screening becomes more widely implemented, we propose examining stage of adoption for screening behavior as a better means to inform future intervention research versus intention to screen because stage of adoption reflects where the individual is in the decision-making process. The proposed conceptual model was developed framed by this key distinction.
Figure 1.
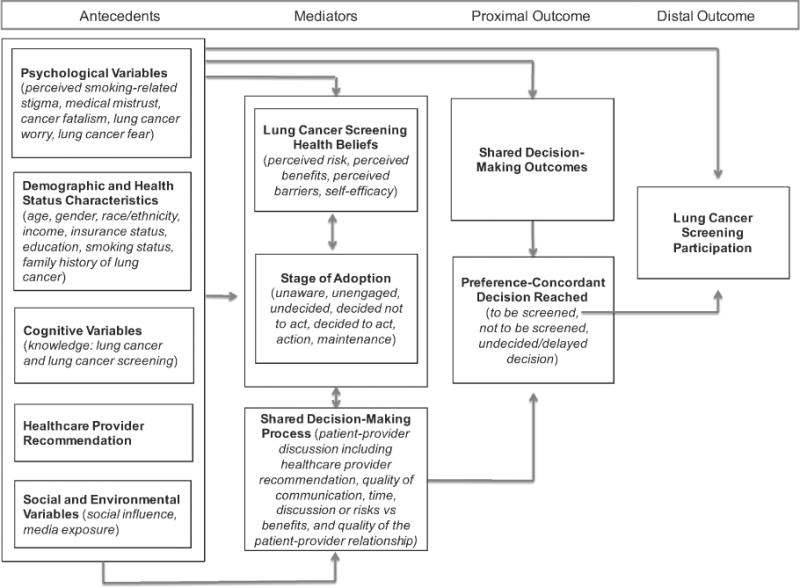
Conceptual model for lung cancer screening participation.
Psychological Variables
A psychological variable is an overarching category that relates to the mental or emotional state of an individual and is conceptualized as an antecedent in the model. Being a smoker adds a unique health status characteristic that may impact lung cancer screening participation differently than other types of cancer screening. These differences may be particularly manifested by psychological variables such as perceived stigma, medical mistrust, lung cancer fatalism, lung cancer worry, and lung cancer fear. Perceived smoking-related stigma has been described by long-term current and former smokers as a key barrier to lung cancer screening (Carter-Harris et al., 2015) as well as negatively associated with timing of medical help-seeking behavior for symptoms suggestive of lung cancer (Carter-Harris, Hermann, Schreiber, Weaver, & Rawl, 2014). Patients diagnosed with lung cancer feared being blamed for the disease because they thought others believed it was self-inflicted by smoking. Several studies in other types of cancer screening have demonstrated that perceived stigma, in some populations, can be a potent barrier to cancer screening (Fang & Baker, 2013; Goldman, Diaz, & Kim, 2009; Ndukwe, Williams, & Sheppard, 2013).
Similarly, in initial qualitative research, medical mistrust was identified as a barrier to lung cancer screening. Individuals described distrust of the health care system, government, and tobacco industry and reported an uncertainty of the value of lung cancer screening comparing “new machines to screen to a scam” (Carter-Harris et al., 2015, p. 7). Medical mistrust has also been positively associated with late-stage lung cancer presentation among ethnic minorities, which may be associated with lung cancer screening participation (Bergamo et al., 2013). Medical mistrust is the belief that the health care system itself and those working within it are untrustworthy. An individual’s mistrust of the health care system and those working within it may impede lung cancer screening participation and requires further examination.
Lung cancer fatalism, as defined in the cancer literature, is the belief that being diagnosed with lung cancer will result in death (Bergamo et al., 2013; Jonnalagadda et al., 2012). Fatalism has been shown to be a barrier to lung cancer screening participation in initial qualitative research but requires further exploration to more fully understand its impact (Bergamo et al., 2013; Jonnalagadda et al., 2012; Patel et al., 2012). In colorectal cancer screening, researchers demonstrated screening participation was 56% higher among individuals with lower cancer fatalism in a prospective study of screening-eligible adults (Miles, Rainbow, & von Wagner, 2011). In addition, findings from key informant interviews and focus group sessions in an urban sample of women screened for breast and cervical cancer suggested fatalism and stigma as major factors influencing the decision to screen versus not screen (Nduke, Williams, & Sheppard, 2013). Researchers have also demonstrated lower odds of having had a mammogram among women with higher fatalistic attitudes about breast cancer (Tolma, Stoner, Li, Kim, & Engelman, 2014). Fatalism is a phenomenon found in individuals making the decision to screen for breast, cervical, and colorectal cancer (Ndukwe et al., 2013; Tolma et al., 2014), and early research has demonstrated its applicability in the context of lung cancer screening (Bergamo et al., 2013; Jonnalagadda et al., 2012; Patel et al., 2012).
Cancer worry is defined as an emotional reaction to the threat of cancer (Hay, Buckley, & Ostroff, 2005). Although cancer worry has not been studied in lung cancer screening, it has predicted screening in other cancers. Moderate levels of cancer worry have predicted breast cancer screening in high-risk women (Diefenbach, Miller, & Daly, 1999; Zhang et al., 2012). Cancer worry about the results of cervical cancer screening was also identified as an important psychosocial barrier to the screening process in an international qualitative study (Teng et al., 2014). Lung cancer worry may be an important psychological variable in the decision to screen or not in lung cancer.
Lung cancer fear refers to the threat of what a lung cancer diagnosis may mean to the individual (Byrne et al., 2008; Patel et al., 2012). Such fear has been shown to be an important barrier to lung cancer screening participation in initial qualitative reports; heightened levels of lung cancer fear have been negatively associated with lung cancer screening participation in current and former smokers (Bergamo et al., 2013; Jonnalagadda et al., 2012; Patel et al., 2012). Similarly, a recent study that explored cancer fear in general found a negative association with cancer screening intentions (Chae, 2015). Our conceptual model proposes psychological variables, specifically perceived smoking-related stigma, medical mistrust, lung cancer fatalism, lung cancer worry, and lung cancer fear, as potential antecedents to the stage of adoption for lung cancer screening, the shared decision-making process and outcomes resulting in a preference-concordant decision, and ultimately, lung cancer screening behavior.
Demographic and Health Status Characteristics
Demographic and health status characteristics have been related to lung and other cancer screening behaviors and are included in the conceptual model as antecedents. Ethnic minorities have been less likely to intend to screen for lung cancer secondary to cost issues (Jonnalagadda et al., 2012), and having health insurance was positively associated with lung cancer screening (Bergamo et al., 2013). Although age, gender, and income have not been specifically assessed in lung cancer screening behavior, researchers have examined these demographic variables in other types of cancer screening. Being older and being a female have both been associated with colorectal cancer screening participation, whereas lower income has been associated with lower likelihood of colorectal cancer screening participation (Mansouri McMillan, Grant, Crighton, & Horgan, 2013). Because it is not yet known if age, gender, and income are associated with lung cancer screening participation, although they have been associated with other types of cancer screening, these variables are included in our model.
Smoking status and family history of lung cancer have been identified qualitatively as potentially influential in lung cancer screening participation. Smoking status has been defined as being a never smoker, current smoker, or former smoker (Leffondré, Abrahamowicz, Siemiatycki, & Rachet, 2002). Studies examining relationships among smoking status and attitudes and intentions toward lung cancer screening showed conflicting results. One study reported veterans who were current or former smokers were more likely to intend to screen for lung cancer (Tanner et al., 2013), whereas another study involving a sample of nonveteran current and former smokers cited negative attitudes toward lung cancer screening participation as a barrier (Patel et al., 2012; Silvestri et al., 2007). Researchers of the study with veterans noted chemical exposures during military service that were associated with an increased cancer risk may have influenced intentions to screen for lung cancer in this population (Tanner et al., 2013).
Similarly, having a family history of lung cancer has been associated with willingness to screen regardless of smoking status (Patel et al., 2012). In a large population-based cohort study, predictors of lung cancer screening were being a current smoker, having smoked a greater number of pack-years, and having a positive family history of lung cancer (Dominioni et al., 2010).
Cognitive Variable
Knowledge has been examined in various studies of cancer screening participation. Although not specifically examined in lung cancer, the cognitive variable of knowledge of lung cancer and knowledge of lung cancer screening may influence lung cancer screening participation. Knowledge of cancer and screening guidelines is an important predictor of screening participation (Tessaro, Mangone, Parkar, & Pawar, 2006) and included as an antecedent in the model. Knowledge about colorectal cancer screening guidelines has predicted positive screening participation, and lack of knowledge about the need to screen was cited as a major barrier to screening participation (Tessaro et al., 2006). Knowledge has also been shown to be important in populations of women screened for breast cancer and was the best predictor of screening adherence in that context (Charkazi et al., 2013).
Health Care Provider Recommendation
Another important variable in cancer screening behavior is receiving a recommendation from a health care provider and, in fact, it is often the most important predictor of all types of cancer screening behaviors (Dominick et al., 2003; Ye, Xu, & Aladesanmi, 2009). Although not specifically studied in lung cancer, one study of 209 military veterans reported that 41.5% of current smokers who were willing to be screened for lung cancer had recently been told by a physician they were at high risk for developing lung cancer (Tanner et al., 2013). In a large study of 3,059 adults age 50 years or older, a health care provider recommendation for colorectal cancer screening was significantly associated with subsequent screening (odds ratio [OR] 5 4.74; Ye et al., 2009). Similarly, a health care provider recommendation was reported as the best predictor for receiving a screening colonoscopy in a recent study of patients who had no prior history of screening (Ramdass, Petraro, Via, Shahrokni, & Nawaz, 2014). Health care provider recommendation is likely to be a significant predictor of lung cancer screening participation and is included as an antecedent in the model.
Social and Environmental Variables
Social and environmental variables are included in the proposed conceptual model as antecedents because they have been shown to predict cancer screening participation in other cancers. Social and environmental variables refer to elements in an individuals’ community including the surroundings in which an individual lives that have the potential to influence their behavior. An individual faced with making a decision about lung cancer screening is likely to also be influenced by social and environmental variables such as social influence and exposure to mass media campaigns (Anderson, Mullins, Siahpush, Spittal, & Wafefield, 2009; de Nooijer, Lechner, & de Vries, 2003; Honda, 2004; Morrell, Perez, Hardy, Cotter, & Bishop, 2010). Social influence, defined as the influence of family and friends on an individual’s behavior (Allen, Sorensen, Stoddard, Peterson, & Colditz, 1999), has predicted intent to screen for breast, colorectal, and prostate cancer (Steadman & Rutter, 2004). In addition, social influence exerted by an individual’s informal support network has predicted adherence to colorectal cancer screening among at-risk Japanese Americans (Honda, 2004).
Media exposure has been included to reflect the potential influence of commercial, print, and social media on cancer screening participation (Anderson et al., 2009; Morrell et al., 2010). Individual exposure to mass media campaigns in Australia to promote cervical cancer awareness resulted in increased screening rates among unscreened, screening-eligible women (Anderson et al., 2009; Morrell et al., 2010). In addition, individual exposure to media has been shown to moderate the relationship between socioeconomic status and fear of cancer and, thus, cancer screening behavior in the United States (Jung, Chan, & Viswanath, 2014).
Lung Cancer Screening Health Beliefs
Health beliefs are included in the proposed conceptual model because they have been shown to predict cancer screening participation in other types of cancer and are included as potential mediators. Health beliefs are primary concepts that have been used to explain why people take preventive actions, screen for certain illnesses, and take actions to control an illness (Champion & Skinner, 2008). Notably, health beliefs are potentially modifiable intervention targets in cancer screening participation and may mediate the relationship between key antecedent variables and the decision to screen, or not, for lung cancer. Perceived risk, perceived benefits, perceived barriers, and self-efficacy are health beliefs that reflect individual beliefs about lung and other cancer screening behaviors (Byrne et al., 2008; Jonnalagadda et al., 2012; Wools, Dapper, & de Leeuw, 2016).
HBM constructs have been reported as important in qualitative reports exploring participation in lung cancer screening (Bergamo et al., 2013; Byrne et al., 2008; Carter-Harris et al., 2015; Jonnalagadda et al., 2012; Patel et al., 2012; Tanner et al., 2013) and predictive in other types of cancer screening (Wools et al., 2015). Perceived risk is conceptually defined as an individuals’ belief in the likelihood they will develop lung cancer (Patel et al., 2012) and has been shown to predict intention to screen for lung cancer (Tanner et al., 2013). In the previously referenced sample of 209 military veterans, smokers were more likely to have been told by a physician and to believe they were at high risk for lung cancer. Nearly all veterans surveyed (92.8%), regardless of smoking status, indicated they would participate in lung cancer screening (Tanner et al., 2013). The investigator reported veterans who had never smoked were more likely to agree to screening (compared to never-smokers in the general population) because of harmful military-associated environmental and occupational exposures. This important variable needs further evaluation in nonveterans eligible for lung cancer screening.
Perceived benefits are conceptually defined as the belief in the efficacy of an advised course of action to reduce risk (Champion & Skinner, 2008). In the context of lung cancer, perceived benefits are the individuals’ beliefs about the positive outcomes associated with lung cancer screening participation. Higher perceived benefits of lung cancer screening were associated with increased willingness to participate in lung cancer screening in the 2013 study with 209 military veterans (Tanner et al., 2013). In addition, focus group participants reported finding lung cancer early, giving peace of mind, and providing a motivation to quit smoking as three perceived benefits of lung cancer screening in a 2015 study (Carter-Harris et al., 2015).
Perceived barriers are conceptually defined as an individual’s belief about the costs (both tangible and psychological) of the advised course of action (Champion & Skinner, 2008). In the context of lung cancer, perceived barriers are a person’s estimation of the level of challenge associated with lung cancer screening participation (Patel et al., 2012). For example, in lung cancer screening, fear and the belief that one is too old to benefit from screening as well as inconvenience have been identified as barriers to participation (Carter-Harris et al., 2015; Patel et al., 2012). In breast cancer screening, concerns about cost (40%), mammogram-related pain (13%), and fear of bad news (13%) were the most commonly reported barriers in a group of underserved women (Fayanju, Kraenzle, Drake, Oka, & Goodman, 2014).
Self-efficacy is conceptually defined as the confidence individuals have in their ability to take action (Champion & Skinner, 2008). In the context of lung cancer, self-efficacy is confidence that one has the ability to perform all tasks related to arranging and completing lung cancer screening. Two studies have found a positive association between intent to screen and an individuals’ confidence about talking with their health care provider about lung cancer screening (Carter-Harris et al., 2015; Jonnalagadda et al., 2012). Self-efficacy has been positively associated with breast and colorectal cancer screening further supporting the association with lung cancer screening participation (Taymoori, Berry, & Roshani, 2014; Wools et al., 2015). In addition, self-efficacy has been examined from a provider perspective. Researchers in one study reported physicians who had higher self-efficacy for counseling their patients about health behavior modifications related to colorectal cancer risk were more likely to recommend colonoscopy; these findings offer support for the potential meditational role for self-efficacy (Honda & Gorin, 2006). Perceived risk, perceived benefits, perceived barriers, and self-efficacy have been included as potential mediators in the proposed model because these variables have predicted participation in other types of cancer screening (Taymoori et al., 2014; Wools et al., 2015) and are likely important in lung cancer screening participation.
Stage of Adoption
Stage of adoption is frequently defined in a transtheoretical model (TTM) context and has been explored extensively in other cancer screening studies (Brenes & Paskett, 2000; Menon et al., 2007; Rawl et al., 2005). However, little is known about stage of adoption for lung cancer screening. Cancer screening decisions can be conceptualized along a continuum from being unaware of the existence of a type of cancer screening and/or guidelines to a decision. Although the TTM has been used to guide numerous health behavior studies of stage of adoption, it does not include a stage to categorize people who have decided not to be screened. Because people may decide to not participate in lung cancer screening after thoroughly weighing their options, the PAPM was determined to be a more appropriate staging theory because it includes this distinct stage (Weinstein et al., 2008). Similar to the TTM, the PAPM is a stage theory that classifies individuals who are initiating health-protective behaviors according to their stage of decision making. The PAPM also proposes strategies that can assist individuals to transition from one stage to the next in the process of implementing the behavior (Weinstein et al., 2008).
The PAPM describes seven stages that reflect both cognitive and behavioral components of adoption of a protective behavior. Individuals in Stage I are unaware of an issue. Individuals in Stage II are aware of but unengaged by the issue. People in Stage III are undecided about acting, whereas those in Stage IV have decided not to act. Those in Stage V have decided to act at some point in the near future. Individuals in Stage VI are acting, and those in Stage VII are in maintenance. Stage of adoption has been shown to be an important predictor and outcome in breast and colorectal cancer screening studies (Costanza et al., 2005; Menon et al., 2007) and is likely to be useful in research on lung cancer screening participation. See Figure 2 for a diagram of the PAPM in the context of lung cancer screening participation.
Figure 2.
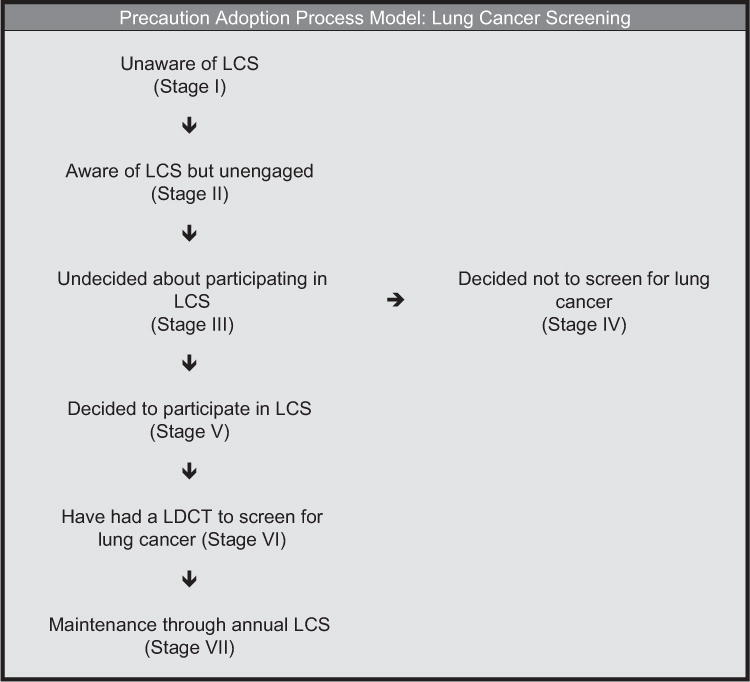
Precaution adoption process model in the context of lung cancer screening.
Shared Decision-Making Process
Shared decision-making, as conceptualized in the USPSTF’s (2014) lung cancer screening recommendation, occurs when the decision to screen results from a thorough discussion between the patient and their health care provider of the possible benefits, limitations, and known and uncertain harms. Shared decision-making inherently involves the health care provider and the patient. Important aspects of the shared decision-making process include the patient—provider relationship, quality of communication, time spent discussing the topic, and content including the patient’s understanding of the health-related decision being addressed. In cancer screening decisions, a health care provider recommendation is an important component of the process. As previously mentioned, preliminary evidence has shown that health care provider recommendation increases lung cancer screening participation (Tanner et al., 2013). Although little is known about the shared decision-making process in the context of lung cancer screening, this process has been shown to be influential in other types of cancer screening (Politi, Studts, & Hayslip, 2012). The shared decision-making process involves the patient—provider discussion and shared decision-making outcomes (discussed in detail in the subsequent text) and influences lung cancer screening participation.
Patient—Provider Discussion
The patient—provider discussion is a critical component of the shared decision-making process, and it is influenced by the health care provider recommendation, quality of communication, time, discussion of risks versus benefits, and quality of the patient—provider relationship. Having a health care provider recommend cancer screening is well known as a predictor of breast and colorectal cancer screening participation (Dominick et al., 2003; Ye et al., 2009), and it is likely to be a critical prerequisite to a patient’s decision to screen for lung cancer. Another key component is the quality of the communication between the health care provider and patient. High-quality communication has been significantly positively correlated with increased colorectal cancer screening participation (Carcaise-Edinboro & Bradley, 2008; Ho, Lai, & Cheung, 2011). Increased time spent with the provider has been correlated with higher cancer screening participation levels (Carcaise-Edinboro & Bradley, 2008; Ho et al., 2011). Finally, risk/benefit discussions and the patient—provider relationship are included as variables in the shared decision-making process and the proposed model because they are identified as influential factors in other types of cancer screening (Dominick et al., 2003; Ye et al., 2009).
It is hypothesized in the proposed conceptual model that the shared decision-making process variables will both influence and be influenced by lung cancer screening health beliefs (perceived risk, perceived benefits, perceived barriers, and self-efficacy) and stage of adoption for lung screening. However, this process may occur over multiple clinical encounters and numerous discussions about lung screening. The double-headed arrows that link lung cancer screening health beliefs to stage of adoption as well as variables (i.e., lung cancer screening health beliefs, stage of adoption) to the shared decision-making process illustrates the likelihood of these variables influencing each other and that multiple patient—provider discussions about lung screening may be needed for the patient to transition forward in stage of adoption toward a decision to screen, or not, for lung cancer.
Shared Decision-Making Outcomes
Shared decision-making outcomes result from an interaction between an individual and their health care provider reflecting the patient—provider discussion and collaboration toward a decision to participate or not in a health care-related course of action. For shared decision-making to occur, the individual must (a) be aware of his or her risk and associated severity; (b) understand his or her risks, benefits, and uncertainties; (c) have weighed his or her values regarding the course of action; and (d) engage in a decision-making process at a level he or she desires and that is comfortable (Sheridan et al., 2004). To enhance lung cancer screening participation, the shared decision-making process should result in a preference-concordant decision, meaning the decision should be consistent with the patient’s preference. Shared decision-making outcomes are conceptualized as a proximal outcome preceding the distal outcome of actual lung cancer screening participation. In addition, the relationships among the proposed antecedents in the conceptual model (psychological variables, demographics and health status characteristics, cognitive variables, health care provider recommendation, and social and environmental variables), shared decision-making outcomes, and lung cancer screening participation are proposed to be mediated by the shared decision-making process variables.
Preference-Concordant Decision
The shared decision-making process can occur over a varying number of encounters between the health care provider and the patient. Ideally, the result of the shared decision-making process and outcomes is a preference-concordant decision. After the patient—provider discussion, a decision to be screened for lung cancer, not to be screened, or an undecided or delayed decision is made. As in other types of cancer screening, the health care provider is instrumental in helping patients understand their risk of developing lung cancer and the benefits and associated harms of lung cancer screening. Ultimately, after patient—provider discussions have occurred and the provider has suggested a course of action, an individual must make a decision. A preference-concordant decision is the result of patients weighing their values regarding the recommendation from the provider to screen, or not, for lung cancer and engaging in a decision-making process at a level that he or she desires and that is comfortable (Sheridan et al., 2004) and conceptualized as a proximal outcome in the model.
DISCUSSION
For the first time, annual LDCT is recommended as an effective screening test for lung cancer based on a 20% relative reduction in mortality among long-term smokers (Aberle et al., 2011). An initial conceptual model was developed to frame an understanding of lung cancer screening participation from the perspective of the individual making the decision to screen or not. This conceptual model has the potential to guide research and enhance understanding of factors associated with lung cancer screening participation, particularly framed in the context of a shared decision-making process. Informed by prior cancer screening research in lung and other cancers and health behavior theory, the proposed conceptual model includes several variables that have either been associated with or predicted screening participation while accounting for the important shared decision-making process that is critical to lung and other cancer screening decisions. It will be essential to test the proposed relationships among all variables in the model including their ability to predict the outcomes of stage of adoption for lung cancer screening participation.
An evidence-based conceptual model to understand lung cancer screening participation offers the opportunity to go beyond simply understanding perceived benefits and barriers to lung cancer screening participation and explicate key relationships associated with screening behavior in this population, which can inform and provide a theoretical foundation on which to develop tailored interventions. For example, a computer-tailored intervention program to support patient—provider discussions about lung screening as part of the shared decision-making process in a clinical encounter can be most effectively designed if first grounded theoretically with a clear understanding of what potentially modifiable variables influence the shared decision-making process as well as lung cancer screening participation. Future research can test the model through path analyses and structural equation modeling and confirm the relative weights of the psychological variables in relation to the HBM variables to indicate if tailored interventions need to tailor on single or multiple variables thereby informing the development of specific intervention content.
Although the development of this conceptual model was informed by theoretical and empirical literature about cancer screening in lung and other cancers, the utility of all variables depicted in the model should be tested. Findings from future research empirically testing the relationships in the model should be continually integrated to inform, refine, and update the proposed model.
CONCLUSION
This article addresses the gap in current knowledge by framing lung cancer screening participation theoretically and from the perspective of the individual making the decision. If the proposed relationships are tested and supported, this model has potential to guide future research focused on designing and testing patient-tailored interventions related to lung cancer screening. Although many of the proposed relationships have been shown to be important in the context of other cancer screening behaviors, to support the development of effective behaviorally focused interventions in this new screening modality, the proposed relationships must be studied in the context of lung cancer screening. We invite colleagues to use the model, test it, and use their results to refine the model to maximize its predictive power and scientific utility.
References
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. http://dx.doi.org/10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Sorensen G, Stoddard AM, Peterson KE, Colditz G. The relationship between social network characteristics and breast cancer screening practices among employed women. Annals of Behavioral Medicine. 1999;21(3):193–200. doi: 10.1007/BF02884833. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts & figures. Atlanta, GA: Author; 2015. Retrieved from http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/ [Google Scholar]
- Anderson J, Mullins RM, Siahpush M, Spittal MJ, Wafefield M. Mass media campaign improves cervical screening across all socio-economic groups. Health Education Research. 2009;24(5):867–875. doi: 10.1093/her/cyp023. [DOI] [PubMed] [Google Scholar]
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology & Health. 1998;13:623–649. [Google Scholar]
- Bergamo C, Lin JJ, Smith C, Lurslurchachai L, Halm EA, Powell CA, Wisnivesky JP. Evaluating beliefs associated with late-stage lung cancer presentation in minorities. Journal of Thoracic Oncology. 2013;8(1):12–18. doi: 10.1097/JTO.0b013e3182762ce4. [DOI] [PubMed] [Google Scholar]
- Boiselle PM. Computed tomography screening for lung cancer. JAMA. 2013;309(11):1163–1170. doi: 10.1001/jama.2012.216988. http://dx.doi.org/10.1001/jama.2012.216988. [DOI] [PubMed] [Google Scholar]
- Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Preventive Medicine. 2000;31(4):410–416. doi: 10.1006/pmed.2000.0729. http://dx.doi.org/10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Medical Decision Making. 2008;28(6):917–925. doi: 10.1177/0272989X08322013. http://dx.doi.org/10.1177/0272989X08322013. [DOI] [PubMed] [Google Scholar]
- Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Medical Care. 2008;46(7):738–745. doi: 10.1097/MLR.0b013e318178935a. http://dx.doi.org/10.1097/MLR.0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- Carpenter CJ. A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Communication. 2010;25:661–669. doi: 10.1080/10410236.2010.521906. http://dx.doi.org/10.1097/MLR0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- Carter-Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: What do long-term smokers know and believe? Health Expectations. 2015 doi: 10.1111/hex.12433. Advance online publication. http://dx.doi.org/10.1111/hex.12433. [DOI] [PMC free article] [PubMed]
- Carter-Harris L, Hermann CP, Schreiber J, Weaver MT, Rawl SM. Lung cancer stigma predicts medical help-seeking behavior in adults with lung cancer. Oncology Nursing Forum. 2014;41(3):E203–E210. doi: 10.1188/14.ONF.E203-E210. http://dx.doi.org/10.1188/14.ONF.E203-E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J. A three-factor cancer-related mental condition model and its relationship with cancer information use, cancer information avoidance, and screening intention. Journal of Health Communication. 2015;20(10):1133–1142. doi: 10.1080/10810730.2015.1018633. http://dx.doi.org/10.1080/10810730.2015.1018633. [DOI] [PubMed] [Google Scholar]
- Champion V, Skinner CS. The health belief model. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: Theory, research, and practice. 4th. San Francisco, CA: Jossey-Bass; 2008. pp. 45–65. [Google Scholar]
- Charkazi A, Samimi A, Razzaghi K, Kouchaki GM, Moodi M, Meirkarimi K, Shahnazi H. Adherence to recommended breast cancer screening in Iranian Turkmen women: The role of knowledge and beliefs. ISRN Preventive Medicine. 2013:1–8. doi: 10.5402/2013/581027. http://dx.doi.org/10.5402/2013/581027. [DOI] [PMC free article] [PubMed]
- Costanza ME, Luckmann R, Stoddard AM, Avrunin JS, White MJ, Stark JR, Rosal MC. Applying a stage model of behavior change to colon cancer screening. Preventive Medicine. 2005;41(3–4):707–719. doi: 10.1016/j.ypmed.2004.12.013. http://dx.doi.org/10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Delmerico J, Hyland A, Celestino P, Reid M, Cummings KM. Patient willingness and barriers to receiving a CT scan for lung cancer screening. Lung Cancer. 2014;84:307–309. doi: 10.1016/j.lungcan.2014.03.003. http://dx.doi.org/10.1016/j.lungcan.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooijer J, Lechner L, de Vries H. Social psychological correlates of paying attention to cancer symptoms and seeking medical help. Social Science & Medicine. 2003;56(5):915–920. doi: 10.1016/s0277-9536(02)00098-9. [DOI] [PubMed] [Google Scholar]
- Diefenbach MA, Miller SM, Daly MB. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychology. 1999;18(5):532–536. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- Dominick KL, Skinner CS, Bastian LA, Bosworth HB, Strigo TS, Rimer BK. Provider characteristics and mammography recommendation among women in their 40s and 50s. Journal of Women’s Health. 2003;12(1):61–71. doi: 10.1089/154099903321154158. http://dx.doi.org/10.1089/154099903321154158. [DOI] [PubMed] [Google Scholar]
- Dominioni L, Rotolo N, Poli A, Paolucci M, Sessa F, D’Ambrosio V, Imperatori A. Self-selection effects in smokers attending lung cancer screening. Journal of Thoracic Oncology. 2010;5(4):428–435. doi: 10.1097/JTO.0b013e3181d2efc7. [DOI] [PubMed] [Google Scholar]
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Barry M. Shared decision making: A model for clinical practice. Journal of General Internal Medicine. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. http://dx.doi.org/10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Baker DL. Barriers and facilitators of cervical cancer screening among women of Hmong origin. Journal of Health Care of the Poor and Underserved. 2013;24(2):540–555. doi: 10.1353/hpu.2013.0067. http://dx.doi.org/10.1353/hpu.2013.0067. [DOI] [PubMed] [Google Scholar]
- Fayanju O, Kraenzle S, Drake BF, Oka M, Goodman MS. Perceived barriers to mammography among underserved women in a Breast Health Center Outreach Program. The American Journal of Surgery. 2014;208(3):425–434. doi: 10.1016/j.amjsurg.2014.03.005. http://dx.doi.org/10.1016/j.amjsurg.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn PM, Betancourt H, Ormseth SR. Culture, emotion, and cancer screening: An integrative framework for investigating health behavior. Annals of Behavioral Medicine. 2011;42(1):79–90. doi: 10.1007/s12160-011-9267-z. http://dx.doi.org/10.1007/s12160-011-9267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R, Diaz JA, Kim I. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qualitative Health Research. 2009;19(11):1559–1568. doi: 10.1177/1049732309349359. http://dx.doi.org/10.1177/1049732309349359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EJ, Rayens MK, Hopenhayn C, Christian WJ. Perceived risk and interest in screening for lung cancer among current and former smokers. Research in Nursing & Health. 2006;29:359–370. doi: 10.1002/nur.20132. http://dx.doi.org/10.1002/nur.20132. [DOI] [PubMed] [Google Scholar]
- Hay JL, Buckley TR, Ostroff JS. The role of cancer worry in cancer screening: A theoretical and empirical review of the literature. Psycho-Oncology. 2005;14(7):517–534. doi: 10.1002/pon.864. http://dx.doi.org/10.1002/pon.864. [DOI] [PubMed] [Google Scholar]
- Ho MY, Lai JY, Cheung WY. The influence of physicians on colorectal cancer screening behavior. Cancer Causes Control. 2011;22(12):1659–1668. doi: 10.1007/s10552-011-9842-4. http://dx.doi.org/10.1007/s10552-011-9842-4. [DOI] [PubMed] [Google Scholar]
- Honda K. A model of perceived risk for colorectal cancer among Japanese Americans. Journal of Cancer Education. 2004;19(4):251–257. doi: 10.1207/s15430154jce1904_14. http://dx.doi.org/10.1207/s15430154jce1904_14. [DOI] [PubMed] [Google Scholar]
- Honda K, Gorin SS. A model of stage of change to recommend colonoscopy among urban primary care physicians. Health Psychology. 2006;25(1):65–73. doi: 10.1037/0278-6133.25.1.65. http://dx.doi.org/10.1037/0278-6133.25.1.65. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda S, Bergamo C, Lin JJ, Lurslurchachai L, Diefenbach M, Smith C, Wisnivesky JP. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012;77(3):526–531. doi: 10.1016/j.lungcan.2012.05.095. http://dx.doi.org/10.1016/j.lungcan.2012.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Chan CKY, Viswanath K. Moderating effects of media exposure on associations between socioeconomic position and cancer worry. Asian Pacific Journal of Cancer Prevention. 2014;15(14):5845–5851. doi: 10.7314/apjcp.2014.15.14.5845. http://dx.doi.org/10.7314/APJCP.2014.15.14.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Marcus PM, Silvestri GA, Han PK, Richards TB, Yuan G, Vernon SW. U.S. primary care physicians’ lung cancer screening beliefs and recommendations. American Journal of Preventive Medicine. 2010;39(5):411–420. doi: 10.1016/j.amepre.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffondré K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: A comparison of different approaches. American Journal of Epidemiology. 2002;156(9):813–823. doi: 10.1093/aje/kwf122. http://dx.doi.org/10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- Lung Cancer Alliance. Screening centers of excellence. 2015 Retrieved from http://www.lungcanceralliance.org/am-i-at-risk/what-do-i-need-to-know-about-screening/where-should-i-be-screened/lung-cancer-screening-centers/
- Mansouri D, McMillan DC, Grant Y, Crighton EM, Horgan PG. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLoS One. 2013;8(6):e66063. doi: 10.1371/journal.pone.0066063. http://dx.doi.org/10.1371/journal.pone.0066063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon U, Champion B, Monahan PO, Daggy J, Hui S, Skinner CS. Health belief model variables as predictors of progression in stage of mammography adoption. American Journal of Health Promotion. 2007;21(4):255–261. doi: 10.4278/0890-1171-21.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A, Rainbow S, von Wagner C. Cancer fatalism and poor self-rated health mediate the association between socioeconomic status and uptake of colorectal cancer screening in England. Cancer Epidemiology Biomarkers & Prevention. 2011;20(10):2132–2140. doi: 10.1158/1055-9965.EPI-11-0453. http://dx.doi.org/10.1158/1055-9965.EPI-11-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell S, Perez DA, Hardy M, Cotter T, Bishop JF. Outcomes from a mass media campaign to promote cervical screening in NSW, Australia. Journal of Epidemiology and Community Health. 2010;64:777–783. doi: 10.1136/jech.2008.084657. [DOI] [PubMed] [Google Scholar]
- Ndukwe E, Williams KP, Sheppard V. Knowledge and perspectives of breast and cervical cancer screening among female African immigrants in the Washington D.C. metropolitan area. Journal of Cancer Education. 2013;28(4):1–10. doi: 10.1007/s13187-013-0521-x. http://dx.doi.org/10.1007/s13187-013-0521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Akporobaro A, Chinyanganya N, Hackshaw A, Seale C, Spiro SG, Griffiths C. Attitudes to participation in a lung cancer screening trial: A qualitative study. Thorax. 2012;67(5):418–425. doi: 10.1136/thoraxjnl-2011-200055. http://dx.doi.org/10.1136/thoraxjnl-2011-200055. [DOI] [PubMed] [Google Scholar]
- Pender NJ. A conceptual model for preventive health behavior. Nursing Outlook. 1975;23(6):385–390. [PubMed] [Google Scholar]
- Politi MC, Studts JL, Hayslip JW. Shared decision making in oncology practice: What do oncologists need to know? The Oncologist. 2012;17(1):91–100. doi: 10.1634/theoncologist.2011-0261. http://dx.doi.org/10.1634/theoncologist.2011-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdass P, Petraro P, Via C, Shahrokni A, Nawaz H. Providers role in colonos-copy screening for colorectal cancer. American Journal of Health Behavior. 2014;38(2):234–244. doi: 10.5993/AJHB.38.2.9. http://dx.doi.org/10.5995/AJHB.38.2.9. [DOI] [PubMed] [Google Scholar]
- Rawl SM, Menon U, Champion VL, May FE, Loehrer P, Sr, Hunter C, Skinner CS. Do benefits and barriers differ by stage of adoption for colorectal cancer screening? Health Education Research. 2005;20(2):137–148. doi: 10.1093/her/cyg110. http://dx.doi.org/10.1093/her/cyg110. [DOI] [PubMed] [Google Scholar]
- Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention: A suggested approach from the U.S. Preventive Services Task Force. American Journal of Preventive Medicine. 2004;26(1):56–66. doi: 10.1016/j.amepre.2003.09.011. http://dx.doi.org/10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62(2):126–130. doi: 10.1136/thx.2005.056036. http://dx.doi.org/10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman L, Rutter DR. Belief importance and the theory of planned behaviour: Comparing modal and ranked modal beliefs in predicting attendance at breast screening. British Journal of Health Psychology. 2004;9(Pt. 4):447–463. doi: 10.1348/1359107042304579. [DOI] [PubMed] [Google Scholar]
- Tanner NT, Egede LE, Shamblin C, Gebregziabher M, Silvestri GA. Attitudes and beliefs toward lung cancer screening among US veterans. Chest. 2013;144:1783–1787. doi: 10.1378/chest.13-0056. http://dx.doi.org/10.1378/chest.13-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymoori P, Berry T, Roshani D. Differences in health beliefs across stage of adoption of mammography in Iranian women. Cancer Nursing. 2013;37(3):208–217. doi: 10.1097/NCC.0b013e31829194bc. http://dx.doi.org/10.1097/NCC.0b013e31829194bc. [DOI] [PubMed] [Google Scholar]
- Teng F, Mitchell SM, Sekikubo M, Biryabarema C, Byamugisha JK, Steinberg M, Ogilvie GS. Understanding the role of embarrassment in gynaecological screening: A qualitative study from the ASPIRE cervical cancer screening project in Uganda. BMJ Open. 2014;4:e004783. doi: 10.1136/bmjopen-2014-004783. http://dx.doi.org/10.1136/bmjopen-2014-004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Preventing Chronic Disease. 2006;3(4):A123. [PMC free article] [PubMed] [Google Scholar]
- Tolma E, Stoner JA, Li J, Kim Y, Engelman K. Predictors of regular mammography use among American Indian women in Oklahoma: A cross-sectional study. BMC Women’s Health. 2014;14(101):1–12. doi: 10.1186/1472-6874-14-101. http://dx.doi.org/10.1186/1472-6874-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Lung cancer: Screening. 2013 Retrieved from http://www.uspreventiveservicestaskforce.org/uspstf/uspslung.htm.
- U.S. Preventive Services Task Force. Final recommendation statement: Lung cancer: Screening, December 2013. 2014 Retrieved from http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening.
- Weinstein ND, Sandman PM, Blalock SJ. The precaution adoption process model. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: Theory, research, and practice. 4th. San Francisco, CA: Jossey-Bass; 2008. pp. 123–147. [Google Scholar]
- Wender R, Fontham ET, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, Smith RA. American Cancer Society lung cancer screening guidelines. Cancer: A Cancer Journal for Clinicians. 2013;63(2):107–117. doi: 10.3322/caac.21172. http://dx.doi.org/10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wools A, Dapper E, de Leeuw JR. Colorectal cancer screening participation: A systematic review. European Journal of Public Health. 2016;26:158–168. doi: 10.1093/eurpub/ckv148. http://dx.doi.org/10.1093/eurpub/cckv148. [DOI] [PubMed] [Google Scholar]
- World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012 Retrieved from http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=lung.
- Ye J, Xu Z, Aladesanmi O. Provider recommendation for colorectal cancer screening: Examining the role of patients’ socioeconomic status and health insurance. Cancer Epidemiology. 2009;33(3–4):207–211. doi: 10.1016/j.canep.2009.07.011. http://dx.doi.org/10.1016/j.canep.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang LR, Chiarelli AM, Glendon G, Mirea L, Knight JA, Andrulis IL, Ritvo P. Worry is good for breast cancer screening: A study of female relatives from the Ontario site of the breast cancer family registry. Journal of Cancer Epidemiology. 2012;2012:545062. doi: 10.1155/2012/545062. http://dx.doi.org/10.1155/2012/545062. [DOI] [PMC free article] [PubMed] [Google Scholar]


