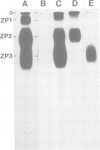
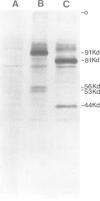
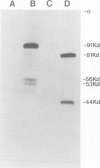
Abstract
During their growth phase, mouse oocytes synthesize and secrete three different glycoproteins, called ZP1, 2 and 3, that constitute the extracellular coat, or zona pellucida, of the oocyte. One of these glycoproteins, ZP3, exhibits properties expected for a sperm receptor. We have now used rabbit antisera that recognize ZP3 to immunoprecipitate [35S]methionine-labeled, intracellular precursors of this glycoprotein from growing oocytes cultured in vitro in the presence or absence of tunicamycin, a drug that prevents addition of N-linked oligosaccharides to nascent polypeptide chains. Electrophoretic analyses of these immunoprecipitates, as well as of immunoprecipitates digested with endo-beta-N-acetylglucosaminidase H (Endo H), indicate that ZP3 is synthesized as a 44,000 mol. wt. polypeptide chain to which either three or four high-mannose-type oligosaccharides are added, resulting in 53,000 and 56,000 mol. wt. ZP3 precursors, respectively. The latter species are converted to mature ZP3 (mol. wt. approximately 80,000) by processing of the high-mannose-type oligosaccharides (Endo H-sensitive) to complex-type oligosaccharides (Endo H-insensitive) prior to ZP3 secretion. The evidence presented reveals that the extreme heterogeneity of mature ZP3, with respect to both mol. wt. and isoelectric point, is partly a consequence of the N-linked oligosaccharides and not the polypeptide chain itself.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleil J. D., Wassarman P. M. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980 Jul;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983 Feb;95(2):317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980 Apr;76(1):185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1029–1033. doi: 10.1073/pnas.77.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Salzmann G. S., Roller R. J., Wassarman P. M. Biosynthesis of the major zona pellucida glycoprotein secreted by oocytes during mammalian oogenesis. Cell. 1982 Dec;31(3 Pt 2):749–759. doi: 10.1016/0092-8674(82)90329-4. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Contribution of oligosaccharide sulfation to the charge heterogeneity of a viral glycoprotein. J Biol Chem. 1982 Aug 10;257(15):9035–9038. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Letourneau G. E., Wassarman P. M. Program of early development in the mammal: changes in patterns and absolute rates of tubulin and total protein synthesis during oogenesis and early embryogenesis in the mouse. Dev Biol. 1979 Feb;68(2):341–359. doi: 10.1016/0012-1606(79)90209-4. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Letourneau G. E., Wassarman P. M. Program of early development in the mammal: changes in the patterns and absolute rates of tubulin and total protein synthesis during oocyte growth in the mouse. Dev Biol. 1979 Nov;73(1):120–133. doi: 10.1016/0012-1606(79)90142-8. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Josefowicz W. J. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol. 1978 May;156(2):209–235. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]