Abstract
Purpose: To investigate the risk factors for recurrence and treatment strategies after patients with hepatocellular carcinoma (HCC) undergo total laparoscopic hepatectomy (LH).
Methods: The study included 109 patients who underwent LH (laparoscopy resection [LR] group, n = 50) or open hepatectomy [OH] (open resection [OR] group, n = 59) for HCC in our hospital between March 2011 and June 2016. Perioperative outcomes, disease recurrence, survival, and risk factors for recurrence were analyzed.
Results: Patient characteristics did not significantly differ between groups. The 1- and 3-year survival rates were 90.7% and 78.1%, respectively, for the LR group and 83.1% and 74.4%, respectively, for the OR group (P = .71). The 1- and 3-year disease-free survival rates were 89.6% and 51.4%, respectively, for the LR group and 84.7% and 59.6%, respectively, for the OR group (P = .935). Tumor size, differentiation, vascular invasion, surgical bleeding, and surgical resection margin were risk factors for tumor recurrence after LH.
Conclusion: LH for HCC did not increase the risk of recurrence compared with OH. Tumor size, differentiation, vascular invasion, surgical bleeding, and surgical resection margin were risk factors for tumor recurrence. Reducing bleeding during surgery and ensuring sufficient surgical margins were the most important measures to reduce postoperative recurrence of HCC.
Keywords: : laparoscopic hepatectomy, hepatocellular carcinoma, survival, strategy, recurrence
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer mortality.1 Surgical resection, liver transplantation, and transarterial chemoembolization are current treatments for HCC, and the main treatment method is surgery.2 Hepatitis virus B or C infection, cirrhosis, the size and number of tumors, encapsulated tumor, pathological grading, and microvascular invasion are closely associated with the postoperative recurrence of HCC.3–7
Laparoscopic liver resection (LLR) was first introduced in the early 1990s by Reich et al.8 and is widely used as a standard technique. Advances in laparoscopic procedures for liver surgery have been slow because of the inherent risk of massive bleeding associated with liver resection. The First International Consensus Conference on Laparoscopic Liver Surgery was held in Louisville, KY in 2008,9 and the 2nd International Consensus Conference on Laparoscopic Liver Resection was held on October, 2014, in Morioka, Japan.10 LLR has increased in frequency, and more than 9000 have been performed worldwide.11,12
Although initial reports describe nonanatomic resection of benign peripheral hepatic lesions, ∼50%–65% of LLRs in large series are now performed to treat cancers, predominantly colorectal liver metastases or HCC.12 Moreover, the number of patients with HCC who undergo laparoscopy resection (LR) has increased steeply over the past 5 years, particularly in Asia and Europe.13–15 Although the use of LLR developed rapidly in recent years, there are concerns about the long-term outcomes of patients with HCC. However, there are no published randomized controlled trials (RCTs) that document the long-term outcomes of patients who undergo LLR, and the available studies conducted at certain centers are limited. Furthermore, compared with open surgery, there are few reports that address whether LLR affects the survival of patients with HCC, recurrence after surgery, as well as the factors that influence postoperative recurrence.
The aim of this study, therefore, was to analyze patients' clinical data to identify the risk factors for recurrence after laparoscopic hepatectomy (LH) and the effects of treatment strategies.
Materials and Methods
Patient selection and data collection
We reviewed the records of patients who underwent curative primary liver resection for HCC at the Affiliated Hospital of Nantong University, China, from March 2011 to June 2016. All operations (laparoscopic and open operations) were performed by the same surgeon, the first LR was undertaken in 2011 at our department. At an early stage, most hepatectomies were performed in the open approach. For tumors located in the periphery of the liver and left lobe as recommended in Louisville, we chose the laparoscopic approach. With the improvement of the operation technique of laparoscopic liver surgery, some cases of the tumor located in segment VII and segment VIII were picked up. Currently, with accumulating enough experience and expanded indication, minor and major resections could be performed; LH took up majority of the operations. If the tumor is located in the vicinity of the hilum or the inferior vena cava or the patient had severe portal hypertension, we prefer open approach to the laparoscopic approach. All operative procedures depended on patients' choice, and after surgery, patients' liver function was assessed according to serum α-fetoprotein (AFP) levels and abdominal computed tomography (CT) or magnetic resonance imaging (MRI).
The selection criteria for LR were as follows: Child–Pugh class A or B, solitary tumor, indocyanine green clearance test (15 minutes) <10%, and combined HCC according to histopathological findings. Exclusion criteria were as follows: uncertain tumor size, involved lymph nodes or extrahepatic metastasis, and large vascular tumor thrombus. The classical definition was used. Minor and major resections involve the removal of ≤2 or ≥3 Couinaud segments, respectively. The final study population comprised 109 patients. The LLR and open resection (OR) groups included 50 and 59 patients, respectively. In the LLR group, 70% (n = 35) and 30% (n = 15) of patients underwent minor and major resections, respectively. In the OR group, 30.5% (n = 18) and 69.5% (n = 41) patients underwent major resections or minor resections, respectively.
Postoperative management and follow-up
All patients were regularly followed to detect recurrence, Follow-up included liver function tests, AFP assays, ultrasonography, and CT every 3 or 4 months. The diagnostic criteria for recurrence were as follows: ultrasonography or CT detected new lesions accompanied by increased AFP levels. However, for new lesions that were not typical, CT and MRI should be used to detect atypical lesions. Recurrence time was defined as the time of surgery to the time of diagnosis of intrahepatic recurrence, and the end point of the study was a patient's death. The average follow-up times for the LLR and OR groups were 22.74 months (range, 1–61 months) and 24 months (range, 1–54 months), respectively.
Statistics
All statistical analyses were performed using SPSS 19.0 version (SPSS, Inc., Chicago, IL). Data are presented as mean and ±standard deviations for variables following normal distribution and were analyzed by Student's t-test. For data following nonnormal distribution, results are expressed as median and range and were compared with Wilcoxon signed rank test. Differences of semiquantitative results were analyzed by Mann–Whitney U-test. Differences of qualitative results were analyzed by χ2 test or Fisher's exact test as appropriate. Survival and recurrence rates were analyzed using the Kaplan–Meier method and differences between the two groups were assessed with the log-rank test. Student's t-test and χ2 test were performed to identify prognostic variables related to recurrence. P < .05 was considered statistically significant.
Results
The LLR and OR groups comprised 50 and 59 patients, respectively. A comparison of baseline and tumor characteristics of the groups is presented in Table 1. There were no significant differences between certain characteristics of each group and are as follows: average age (55.18 ± 10.9 vs. 55.39 ± 9.2 years, P > .05), number of patients with liver cirrhoses (43/50 vs. 50/59, P > .05), Child–Pugh grade A/B (44/6 vs. 53/6, P > .05), average body mass index (BMI) (23.49 ± 2.78 vs. 23.46 ± 2.96 Kg/m2, P > .05), and tumor size (3.38 ± 1.99 vs. 4.03 ± 2.67 cm, P > .05). The location of the tumor differed significantly between groups (P < .05), and the OR group underwent more resections in segments II and III (15 vs. 2, P < .05); however, no tumors were present on the caudal lobe in the LLR group. There were no significant differences in postoperative mortality between groups for blood loss and surgical margins (P > .05), although the LLR group lost less blood during minor resection (303.22 ± 160.79 vs. 431.15 ± 80.21 ml, P > .05) (Table 6).
Table 1.
Clinicopathological Data for the Laparoscopic Resection Group and the Open Resection Group
| Laparoscopic group (n = 50) | Open group (n = 59) | P | |
|---|---|---|---|
| Gender male/female | 35/15 | 46/13 | .384 |
| Age (years) | 55.18 ± 10.9 | 55.39 ± 9.2 | .914 |
| Cirrhoses | 43/50 | 50/59 | .854 |
| BMI | 23.49 ± 2.78 | 23.46 ± 2.96 | .945 |
| Child–Pugh grade A/B | 44/6 | 53/6 | .761 |
| Tumor size (cm) | 3.38 ± 1.99 | 4.03 ± 2.67 | .159 |
| Histology | |||
| Well differentiated | 7 | 10 | .638 |
| Moderately differentiated | 30 | 38 | |
| Poorly differentiated | 13 | 11 | |
| Location | |||
| Left lobe | 18 | 7 | .003 |
| Segments II and III | 15 | 2 | .008 |
| Segment IV | 3 | 5 | |
| Right lobe | 32 | 52 | .03 |
| Segment V | 2 | 10 | |
| Segment VI | 6 | 8 | .046 |
| Segment VII | 14 | 18 | |
| Segment VIII | 10 | 14 | |
| The caudal lobe | 0 | 2 | |
BMI, body mass index.
Table 6.
Intraoperative Data and Surgical Results in Minor Resections
| Laparoscopic group (n = 35) | Open group (n = 41) | P | |
|---|---|---|---|
| Blood loss (mL) | 303.22 ± 160.79 | 431.15 ± 80.21 | .001 |
| Surgical margin (cm) | 1.1 ± 0.49 | 1.2 ± 0.69 | .442 |
There were no significant differences in postoperative mortality between groups (P > .05), the time required for surgery was shorter for the LLR group (152.97 ± 40.85 vs. 176.2 ± 70.84 min, P = .035), and the LLR group was hospitalized for fewer days (13 vs. 16 days, P < .05). At least one postoperative complication was observed in 19% (n = 9) and 37.3% (n = 22) of the patients in the LLR and OR groups, respectively (P = .033). None of the patients in either group underwent repeat surgery, and 2 patients in the OR group were readmitted because of abdominal pain (Table 2).
Table 2.
The Perioperative Outcomes of the Laparoscopic Resection Group and the Open Resection Group
| Laparoscopic group (n = 50) | Open group (n = 59) | P | |
|---|---|---|---|
| Operation time (min) | 176.2 ± 70.84 | 152.97 ± 40.85 | .035 |
| Hospital stay (days) | 13 (8–18) | 16 (8–28) | <.001 |
| Reoperation (90 days), n (%) | 0 | 0 | |
| Readmission (90 days), n (%) | 0 | 2 (3.3) | .189 |
| Postoperative complications | 9 (18%) | 22 (37.3%) | .033 |
| Ascites | 6 | 11 | |
| Pleural effusion | 5 | 9 | |
| Bile leak | 0 | 0 | |
| Liver failure | 0 | 3 | |
| Surgical site infection | 0 | 2 | |
| Bleeding | 0 | 0 | |
| Postoperative mortality, n (%) | |||
| 30 days | 1 (2) | 1 (1.7) | .906 |
| 60 days | 1 (2) | 2 (3.3) | .65 |
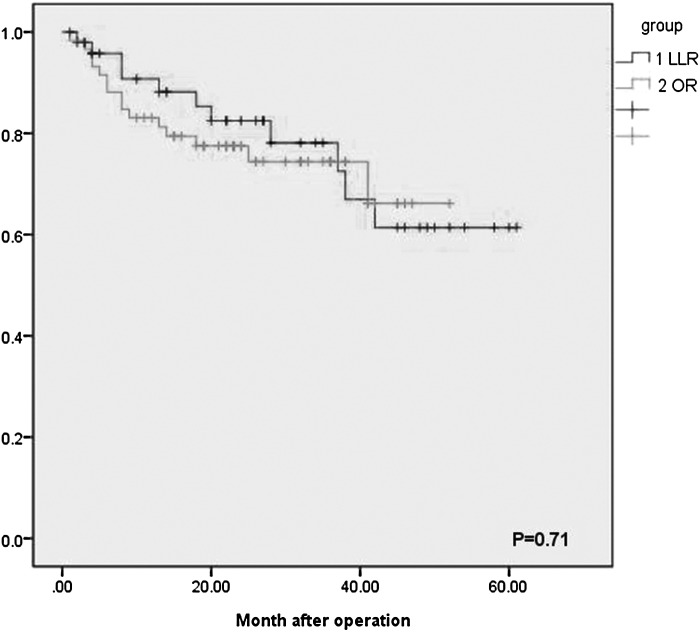
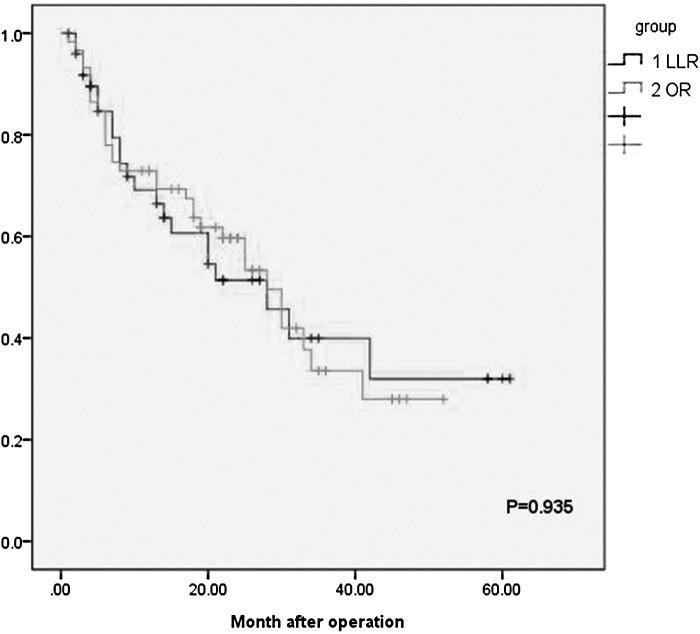
Table 3 compares the long-term outcomes of the groups. The median follow-up periods were 25.04 and 24.08 months in the LLR and OR groups, respectively (P = .814). The 1- and 3-year overall survival (OS) rates were 90.7% and 78.1% in the LLR group, respectively, and 83.1% and 74.4%, respectively, in the open group (P = .764) (Fig. 1). The 1- and 3-year disease-free survival (DFS) rates were 89.6% and 51.4% in the LLR group, respectively, and 84.7% and 59.6% in the OR group, respectively (P = .935) (Fig. 2). Disease recurred during follow-up in 16 (34%) and 23 (39%) patients in the LLR and OR groups, respectively (P = .548). Mean recurrence times in the LLR and OR groups were not significantly different (9.1 ± 5.9 months and 14.8 ± 10.76 months, respectively, P > .05). Intrahepatic recurrence was the main site, 1 patient in the LLR group had portal-site metastasis and 2 patients had omental metastases. Other extrahepatic metastases were mainly detected in the lungs, adrenal glands, and brain, and did not differ significantly between groups.
Table 3.
The Long-Term Outcomes of Two Groups
| Laparoscopic group (n = 50) | Open group (n = 59) | P | |
|---|---|---|---|
| The mean follow-up time (months) | 25.04 ± 18.4 (1–61) | 24.08 ± 13.1 (1–52) | .814 |
| Tumor recurrence | 16 (34%) | 23 (39%) | .548 |
| Time to recurrence (median) | 9.1 ± 5.9 | 14.8 ± 10.76 | .062 |
| Recurrence site | |||
| Intrahepatic | 16/16 (100%) | 23/23 (100%) | .449 |
| Distant | 5/16 | 5/23 | |
| Port-site metastasis | 1 | 0 | |
| Ometum implanting | 2 | 0 | |
| Lung | 1 | 4 | |
| Adrenal | 0 | 2 | |
| Brain | 1 | 1 | |
| Total mortality | 22% | 25.4% | .764 |
| 1 year OS | 90.7% | 83.1% | |
| 3 year OS | 78.1% | 74.4% | .71 |
| 1 year DFS | 89.6% | 84.7% | |
| 3 year DFS | 51.4% | 59.6% | .935 |
DFS, disease-free survival; OS, overall survival.
FIG. 1.
Overall survival in the laparoscopic group and the open group. LLR, laparoscopic liver resection; OR, open resection.
FIG. 2.
Disease-free survival in the laparoscopic group and the open group. LLR, laparoscopic liver resection; OR, open resection.
The LLR group was divided into recurrence and nonrecurrence groups (Table 4). There were no significant differences between groups in age, sex, BMI, cirrhosis, or Child–Pugh grade A/B. The mean estimated blood loss in the recurrence and nonrecurrence groups was 433.12 and 326.76 mL, respectively, (P = .02). The mean surgical time of the recurrence group was 188.23 minutes and that of the nonrecurrence group was 150.63 minutes (P = .08). The number of vascular invasions in the recurrence and nonrecurrence groups was 6 and 2, respectively (P = .001). In the recurrence and nonrecurrence groups, tumor size (2.62 ± 4.64 cm vs. 2.79 ± 1.27 cm, respectively, P = .02) and degree of differentiation (P = .025) differed significantly. The mean surgical margins in the recurrence and nonrecurrence groups differed significantly (0.8 and 1.41, respectively, P = .014). Table 5 compares the groups' outcomes after major resection. There were no significant differences between groups for blood loss and surgical margins (P > .05), although the LLR group lost less blood during minor resection (303.22 ± 160.79 vs. 431.15 ± 80.21 ml, P > .05) (Table 6).
Table 4.
Univariate Analysis of Factors Associated with Recurrence After Laparoscopic Hepatectomy
| Recurrence (n = 16) | No recurrence (n = 34) | P | |
|---|---|---|---|
| Gender male/female | 13/3 | 20/14 | .118 |
| Age (years) | 57.23 ± 11.18 | 50.81 ± 9.08 | .051 |
| Cirrhoses | 14 | 27 | 1 |
| Child–Pugh grade A/B | 1/15 | 2/32 | .959 |
| Diabetes mellitus | 2/14 | 2/32 | .421 |
| Surgical procedure time (min) | 188.23 ± 80.52 | 150.63 ± 33.26 | .08 |
| Blood loss (mL) | 433.12 ± 134.75 | 326.76 ± 156.55 | .02 |
| Vascular invasion | 6 | 2 | .001 |
| Tumor size (cm) | 4.64 ± 2.62 | 2.79 ± 1.27 | .02 |
| Histology: poorly differentiated | 7 | 5 | .025 |
| Location | |||
| Left lobe | |||
| Segments II and III | 5 | 12 | .778 |
| Segment IV | 0 | 3 | .22 |
| Right lobe | |||
| Segment V | 1 | 1 | .578 |
| Segment VI | 3 | 2 | .578 |
| Segment VII | 5 | 9 | .726 |
| Segment VIII | 2 | 7 | .487 |
| Surgical margin (cm) | 0.8 ± 0.27 | 1.41 ± 0.64 | .001 |
| <1 cm (n) | 10 | 9 | .014 |
Table 5.
Intraoperative Data and Surgical Results in Major Resections
| Laparoscopic group (n = 15) | Open group (n = 18) | P | |
|---|---|---|---|
| Blood loss (mL) | 498.67 ± 102.39 | 521.51 ± 81.51 | .411 |
| Surgical margin (cm) | 1.48 ± 0.81 | 1.49 ± 0.63 | .977 |
Discussion
The use of LLR has increased in frequency worldwide because of the accumulating experience of surgeons and improvements in instrumentation.15 Furthermore, the application of LLR has expanded to include minor16,17 and major18–21 resections, robotic hepatectomy,22 anatomical resection,23 and donor hepatectomy.24 The number of patients with HCC undergoing LLR has increased steeply over the past 5 years, particularly in Asia and Europe.13–15 After achieving better short-term outcomes, there remain concerns about long-term outcomes such as survival and recurrence. Moreover, LLR is challenging to administer to patients with liver cirrhoses. Therefore, the identification of risk factors for recurrence after LH will likely reduce the recurrence rate and increase long-term survival.
The long-term outcomes of patients with HCC who underwent LH have been reported.25–27 Cheung et al.28 retrospectively analyzed 32 patients with HCC and found that the 1- and 3-year survival rates were 96.6% and 95.2% in the LLR group, respectively, and 87.5% and 72.9% in the OR group, respectively (P = .142). The 1- and 3-year DFS rates of the LLR group were 87.3% and 63.5%, respectively, and 72.6% and 50%, respectively, in the OS group (P = .086).28 Huang and colleagues29 retrospectively analyzed 59 patients with HCC and found no significant difference in 5-year DFS and OS between them. According to TNM staging, OS and DFS did not differ significantly between the two groups, thereby indicating oncological safety. Memeo et al.30 conducted a retrospective case–control study of 45 patients with HCC and found no significant differences between the comparisons, and are as follows: the 1- and 5-year survival rates of the LLR group were 88% and 59%, respectively, and were 63% and 44%, respectively, for the OR group. The DFS rates were 80% and 19%, respectively, for the LLR group and were 60% and 23%, respectively, for the OR group. Consistent with these data, in this study, there were no significant differences between the outcomes of the groups, and are as follows: the 1- and 3-year survival rates of the LLR group were 81.75% and 73.1%, respectively, and the DFS rates were 71.27% and 55.63%, respectively. The 1- and 3-year survival rates of the OR group were 83.05% and 74.4%, respectively, and the DFS rates were 80.04% and 45.2%, respectively, (P > .05) (Table 3).
Although available data were acquired only from case–control studies, cohort studies, or case reports, no RCTs are published. Moreover, research conducted by several centers on the treatment of patients with HCC who underwent LH shows that there is no significant increase in the rates of recurrence and mortality and that LH is a safe and effective therapy for HCC. The number of patients reported is limited, and more data and longer follow-up are required.
In this study, HCC recurred during follow-up in 16 (34%) and 23 (39%) patients in the LRR and OR groups, respectively. Furthermore, the mean recurrence times were not significantly different between the LRR and OR groups (9.1 ± 5.9 and 14.8 ± 10.76 months, respectively, P > .05). The main site of recurrence was intrahepatic, 1 patient in the LLR group had portal-site metastasis and 2 patients had omental metastases. Tumor size, degree of differentiation, vascular invasion, intraoperative bleeding, and surgical margin were the main risk factors for recurrence after LH. These characteristics are determined by the malignant phenotype of a tumor; however, the surgeon must minimize intraoperative bleeding and ensure a sufficient surgical margin.
Owing to the limitation of anatomical space and the lack of direct access to the tumor, it is difficult to control bleeding and to create an appropriate surgical margin during laparoscopic surgery. Using auxiliary equipment to improve the surgical technique, we accumulated experience that helps us prevent and control intraoperative bleeding. The classical definition was used. Minor and major resections involved the removal of ≤2 or ≥3 Couinaud segments, respectively. There was no significant difference in intraoperative bleeding between groups, and the prevention and control of bleeding during surgery are the key to reduce intraoperative bleeding.
The occlusion of hemihepatic blood flow and selective laparoscopy can reduce hepatic ischemia–reperfusion injury, which reduces the mortality rate.31 Therefore, for patients with insufficient liver function, the remnant volume of the liver must be preserved, and we should consider selective occlusion of the hemihepatic and portal vein blood flow. For minor resections, the resection volume is limited. Therefore, dissecting the porta hepatis is not required, and the blood flow occlusion can be predetermined to prevent bleeding.
Ratti et al.32 suggest that selecting the appropriate surgical approach can improve results and that liver texture, tumor size, and the relationship with the hepatic vein should be considered. Here we chose the anterior approach to reduce tumor rupture and bleeding for deep, large tumors with a maximum diameter of 10 cm. For right- posterior-lobe tumors, we mobilized the liver using a lateral approach as the first step and then transected the liver. Furthermore, preoperative simulation and navigation can enhance the surgical management of endoscopic resection to reduce surgical time and intraoperative bleeding.33 We applied preoperative three-dimensional (3D) reconstruction, 3D printing, and intraoperative ultrasonography to determine the location of the tumor surrounding the blood vessels before parenchymal transection because of the superficial layer of the liver parenchyma without a large pulse tube, and we used an ultrasonic scalpel to transect the liver parenchyma.
Deeper transection should be meticulously performed by exposing intraparenchymal structures. Hemostasis is usually achieved using bipolar cautery for vessels ≤2 mm and with vessel sealing devices or clips for vessels 3–7 mm. Locked clips or staplers are used for vessels >7 mm. As in open surgery, some authors recommend the use of staplers for parenchymal transection.34 This is an efficient and expeditious technique. However, some consider that it lacks precision and cannot identify divided structures. Almost all authors report using staplers to secure and divide major vessels such as the main hepatic veins or portal vein branches as well as the segmental Glissonian pedicles. Therefore, multiple surgical implements are frequently chosen if intraoperative bleeding is excessive. The CO2 pressure in the pneumoperitoneum is generally established at 10–14 mmHg,35–37 and this provides for sufficient control of back bleeding during liver transection. Low central venous pressure (<5 mmHg) should be used during LLR. In cases of severe bleeding, increasing the pneumoperitoneum pressure and decreasing the airway pressure using a brief pause in artificial ventilation38 are maneuvers that can be used to decrease back bleeding. Although there are no data indicating the pneumoperitoneum pressure that should be used to decrease back bleeding, the experts who participated in the Morioka Conferece10 recommended 16–20 mmHg. Careful inspection after decreasing the pneumoperitoneum pressure should be performed routinely, and suturing skills are particularly important for performing LLR.
The surgical margin is determined by visual evaluation of a surgical resection, particularly if it is measured using the same pathological laboratory data.39 For example, the positive surgical margin rates in the OR and LR groups were not significantly different for tumors close to the main vasculature. Van der Poel and colleagues40 analyzed 37 patients undergoing LLR to treat the lesion from the main blood vessels within 2 cm (portal vein, left hepatic artery, and the first branch of the inferior vena cava) and found that R0 resection reached 92%, and the 30-day postoperative mortality rate was 0%, indicating feasibility and safety. Here, the surgical margins of the LR group were not inferior to those of the OR group who underwent minor and major resections.
The best surgical margin is still controversial, and the Japanese guidelines recommend no tumor exposure,41 although some recommend 5 mm.42 Moreover, some studies found that a margin >1 cm can reduce the recurrence rate of HCC.39 Here we show that the best surgical margins should reach >1 cm (Table 5). Preoperative simulation system can help measure residual liver volume, and direct observation of the tumor can determine the surgical margin required for hepatectomy.43 To ensure the safety of the surgical margin and reduce unnecessary exposure of the tumor, ultrasonography may help determine tumor size, accurate positioning, and identification of the surrounding blood vessels to determine the cutting plane. Some studies found that the vasculature could be retained within 1 cm.41 For tumors within 1 cm of the main vasculature, we recommend not preserving the vascular or not to recommend laparoscopic surgery.
For minor resection, if the tumor is located in the superior liver, ultrasonography should be used to ensure adequate surgical margins for nonanatomical liver resection as well as for the tumors >3 cm from the surface of the liver. Depending on the size and location of tumor, the adjacent blood vessels, combined with the experience of the surgeon to judge the difficulty, a determination can be made to implement nonanatomical or segment resection. In HCC, micrometastases disseminate through portal venous branches, and therefore anatomic resection is preferred versus nonanatomic resection when liver resection is performed with curative intent. Thus, an anatomic liver resection with a wider resection margin theoretically offers a greater potential for cure.
Anatomical resection refers to parenchymal-preserving resections of portal territories, including sectionectomy, segmentectomy, and subsegmentectomy.23,44,45 These are complex resections that require identification of anatomical boundaries. These boundaries rely on external landmarks, intraoperative ultrasound, and selective clamping using the Glissonian approach,46 which applies to LLR and represents a difficult challenge. Table 1 shows that the S1 segment was not reported, although there is a center to complete surgery of all liver segments,47 although the experience of the surgeon is required to select the appropriate patient.
Recurrence was mainly intrahepatic, followed by metastasis to the lung, bone, brain, and adrenal glands (Table 3). Here, the LLR group experienced portal site and omentum metastases, which may be explained by the lack of surgical experience that may have caused excessive exposure and compression of the tumor. For example, if we did not sufficiently wash the peritoneum postoperatively, the tumor may have spread and recurred. With the improvement of surgical technique, we recommend minimal tumor manipulation, resecting the tumor with adequate margins, peritoneal lavage with heparin, or with cytocidal solutions to avoid the adhesion of free cells, use of protective bags for tissue retrieval, avoiding CO2 leaks and sudden desufflations, use of heated and humidified CO2, exsufflation of the peritoneum before removal of the ports, drainage placement (if required) before deflating the abdomen, irrigation of the ports with heparin or povidone–iodine solution before removal, and closure of all abdominal layers including the peritoneum. Similar circumstances did not reappear during follow-up.
Compared with the OR group patients, the LLR group patients spent more time in surgery, lost less blood (in minor resections), were hospitalized for a shorter stay, and experienced fewer postoperative complications. However, different procedures were performed depending on the location of tumor, and there were limitations to our retrospective study. For example, the perioperative and the long-term outcomes might have been influenced by the location of more tumors in the left lobe and fewer tumors in the right lobe in the LR group than those in the OR group (P < .05). Moreover, due to the relatively small number of patients with HCC, a type II error may have influenced our statistical analysis. To address these limitations, we are accumulating more patients for future studies.
In summary, LLR compared with open surgery for HCC did not affect long-term survival and recurrence. Nevertheless, randomized studies of more patients treated at multiple centers are required, and laparoscopic surgery must be standardized. Reducing bleeding and ensuring the surgical margin are the most important factors for reducing recurrence after LLR.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81070360) and clinical medical science and technology of Jiangsu Technical Department (BL2014060); the project “medical leading talent and innovation team” (LJ201134) of Jiangsu Province; “CyanEngineering” of Jiangsu Province ([2010]27); and graduate innovation project (SJLX15_0644) of Jiangsu Education Department.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004;127(5 Suppl 1):S5–S16 [DOI] [PubMed] [Google Scholar]
- 2.Jibiki M, Inoue Y, Sugano N, Iwai T, Katou T. Tumor thrombectomy without bypass for low-grade malignant tumors extending into the inferior vena cava: Report of two cases. Surg Today 2006;36:465–469 [DOI] [PubMed] [Google Scholar]
- 3.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: Significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer 2004;112:44–50 [DOI] [PubMed] [Google Scholar]
- 4.Collette S, Bonnetain F, Paoletti X, et al. Prognosis of advanced hepatocellular carcinoma: Comparison of three staging systems in two French clinical trials. Ann Oncol 2008;19:1117–1126 [DOI] [PubMed] [Google Scholar]
- 5.Toshikuni N, Takuma Y, Goto T, Yamamoto H. Prognostic factors in hepatitis C patients with a single small hepatocellular carcinoma after radiofrequency ablation. Hepatogastroenterology 2012;59:2361–2366 [DOI] [PubMed] [Google Scholar]
- 6.Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB 2015;17:422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enooku K, Uranbileg B, Ikeda H, et al. Higher LPA2 and LPA6 mRNA levels in hepatocellular carcinoma are associated with poorer differentiation, microvascular invasion and earlier recurrence with higher serum autotaxin levels. PLoS One 2016;11:e0161825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78(5 Pt 2):956–958 [PubMed] [Google Scholar]
- 9.Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825–830 [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619–629 [DOI] [PubMed] [Google Scholar]
- 11.Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 2016;263:761–777 [DOI] [PubMed] [Google Scholar]
- 12.Cheek SM, Sucandy I, Tsung A, Marsh JW, Geller DA. Evidence supporting laparoscopic major hepatectomy. J Hepatobiliary Pancreat Sci 2016;23:257–259 [DOI] [PubMed] [Google Scholar]
- 13.Goumard C, Farges O, Laurent A, et al. An update on laparoscopic liver resection: The French Hepato-Bilio-Pancreatic Surgery Association statement. J Vis Surg 2015;152:107–112 [DOI] [PubMed] [Google Scholar]
- 14.Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: A European experience. J Am Coll Surg 2010;211:16–23 [DOI] [PubMed] [Google Scholar]
- 15.Coelho FF, Kruger JA, Fonseca GM, et al. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg 2016;8:5–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: Long-term survival and role of secondary liver transplantation. Ann Surg 2009;250:738–746 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Nitta H, Otsuka K, Takahara T, Nishizuka S, Wakabayashi G. Ten-year experience of totally laparoscopic liver resection in a single institution. Br J Surg 2009;96:274–279 [DOI] [PubMed] [Google Scholar]
- 18.Dagher I, O'Rourke N, Geller DA, et al. Laparoscopic major hepatectomy: An evolution in standard of care. Ann Surg 2009;250:856–860 [DOI] [PubMed] [Google Scholar]
- 19.Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: A systematic literature review and comparison of 3 techniques. Ann Surg 2013;257:205–213 [DOI] [PubMed] [Google Scholar]
- 20.Belli G, Gayet B, Han HS, et al. Laparoscopic left hemihepatectomy a consideration for acceptance as standard of care. Surg Endosc 2013;27:2721–2726 [DOI] [PubMed] [Google Scholar]
- 21.Dagher I, Gayet B, Tzanis D, et al. International experience for laparoscopic major liver resection. J Hepatobiliary Pancreat Sci 2014;21:732–736 [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi G, Sasaki A, Nishizuka S, Furukawa T, Kitajima M. Our initial experience with robotic hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci 2011;18:481–487 [DOI] [PubMed] [Google Scholar]
- 23.Ho CM, Wakabayashi G, Nitta H, et al. Total laparoscopic limited anatomical resection for centrally located hepatocellular carcinoma in cirrhotic liver. Surg Endosc 2013;27:1820–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahara T, Wakabayashi G, Hasegawa Y, Nitta H. Minimally invasive donor hepatectomy: Evolution from hybrid to pure laparoscopic techniques. Ann Surg 2015;261:e3–e4 [DOI] [PubMed] [Google Scholar]
- 25.Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721–727 [DOI] [PubMed] [Google Scholar]
- 26.Truant S, Bouras AF, Hebbar M, et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: A case-matched study. Surg Endosc 2011;25:3668–3677 [DOI] [PubMed] [Google Scholar]
- 27.Yamashita Y, Ikeda T, Kurihara T, et al. Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: A single-center experience over a 10-year period. J Am Coll Surg 2014;219:1117–1123 [DOI] [PubMed] [Google Scholar]
- 28.Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A single-center experience. Ann Surg 2013;257:506–511 [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Liu L, Zhang Q, et al. Laparoscopic versus open hepatectomy for hepatocellular carcinoma: Long-term outcomes. J BUON 2016;21:135–141 [PubMed] [Google Scholar]
- 30.Memeo R, de'Angelis N, Compagnon P, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World J Surg 2014;38:2919–2926 [DOI] [PubMed] [Google Scholar]
- 31.Tan J, Tan Y, Zhu Y, et al. Perioperative analysis of laparoscopic liver resection with different methods of hepatic inflow occlusion. J Laparoendosc Adv Surg Tech A 2012;22:343–348 [DOI] [PubMed] [Google Scholar]
- 32.Ratti F, Cipriani F, Catena M, Paganelli M, Aldrighetti L. Approach to hepatocaval confluence during laparoscopic right hepatectomy: Three variations on a theme. Surg Endosc 2017;31:949. [DOI] [PubMed] [Google Scholar]
- 33.Marubashi S, Gotoh K, Akita H, et al. Navigation guidance using polyglycolic acid felt in pure laparoscopic partial hepatectomy. Surg Innov 2015;22:355–359 [DOI] [PubMed] [Google Scholar]
- 34.McCall J. Evaluation of stapler hepatectomy in laparoscopic liver resection. HPB 2014;16:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruger JA, Fonseca GM, Coelho FF, Jeismann V, Herman P. Laparoscopic right hepatectomy for cirrhotic patients: Takasaki's Hilar Control and Caudal Approach. Ann Surg Oncol 2017;24:558–559 [DOI] [PubMed] [Google Scholar]
- 36.Tranchart H, Di Giuro G, Lainas P, et al. Laparoscopic liver resection with selective prior vascular control. Am J Surg 2013;205:8–14 [DOI] [PubMed] [Google Scholar]
- 37.Jayaraman S, Khakhar A, Yang H, Bainbridge D, Quan D. The association between central venous pressure, pneumoperitoneum, and venous carbon dioxide embolism in laparoscopic hepatectomy. Surg Endosc 2009;23:2369–2373 [DOI] [PubMed] [Google Scholar]
- 38.Honda G, Kurata M, Okuda Y, et al. Totally laparoscopic hepatectomy exposing the major vessels. J Hepatobiliary Pancreat Sci 2013;20:435–440 [DOI] [PubMed] [Google Scholar]
- 39.Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial. Ann Surg 2007;245:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu Hilal M, van der Poel MJ, Samim M, et al. Laparoscopic liver resection for lesions adjacent to major vasculature: Feasibility, safety and oncological efficiency. J Gastrointest Surg 2015;19:692–698 [DOI] [PubMed] [Google Scholar]
- 41.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 2003;26:142–147 [DOI] [PubMed] [Google Scholar]
- 42.Postriganova N, Kazaryan AM, Rosok BI, Fretland A, Barkhatov L, Edwin B. Margin status after laparoscopic resection of colorectal liver metastases: Does a narrow resection margin have an influence on survival and local recurrence? HPB 2014;16:822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P., 2nd International Consensus Conference on Laparoscopic Liver Resection Group. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: Current status and future perspectives. J Hepatobiliary Pancreat Sci 2015;22:353–362 [DOI] [PubMed] [Google Scholar]
- 44.Herman P, Kruger J, Lupinacci R, Coelho F, Perini M. Laparoscopic bisegmentectomy 6 and 7 using a Glissonian approach and a half-Pringle maneuver. Surg Endosc 2013;27:1840–1841 [DOI] [PubMed] [Google Scholar]
- 45.Machado MA, Surjan RC, Makdissi FF. Intrahepatic glissonian approach for single-port laparoscopic liver resection. J Laparoendosc Adv Surg Tech A 2014;24:534–537 [DOI] [PubMed] [Google Scholar]
- 46.Choi Y, Han HS, Sultan AM, Yoon YS, Cho JY. Glissonean pedicle approach in laparoscopic anatomical liver resection. Hepatogastroenterology 2014;61:2317–2320 [PubMed] [Google Scholar]
- 47.Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: From segment I to VIII. Ann Surg 2012;256:959–964 [DOI] [PubMed] [Google Scholar]