Abstract
Prostate cancer is the second most common cancer in men worldwide. While clinicians commonly see metastases to the bones and lymph nodes, it may infrequently spread to more uncommon locations. We report an unusual case of an 83-year-old patient with previously treated prostate adenocarcinoma who presents with symptomatic metastases to the testis and brain in the absence of widely disseminated disease. This case report highlights the importance of including metastatic disease in the differential for patients with a history of prostate cancer and a newly discovered mass until an evaluation of the tissue can be performed.
INTRODUCTION
Prostate cancer (pca) is the most commonly diagnosed visceral cancer in the United States and the second most common cancer in men worldwide [1, 2]. Common sites of metastasis include the bone and lymph nodes, while less common sites include the liver and lung [3]. Nonetheless, pca is capable of spreading to any organ, including those deemed resistant to the establishment of pca metastases.
CASE REPORT
An 83-year-old Caucasian man presented with right scrotal pain. Scrotal ultrasound demonstrated a 4.2 cm solid mass in the right testicle concerning for malignancy. Three years prior, the patient had been diagnosed with pca via biopsy demonstrating a Gleason 8 (4 + 4) prostate adenocarcinoma. He had undergone radiation therapy with curative intent. His serum prostate-specific antigen (PSA) 27 months post-radiation was 60.5 ng/mL (normal range: 0–4 ng/mL), though serial bone scans remained negative. When the patient developed the right testicular mass 3 years post-treatment, his PSA was 319 ng/mL. The patient underwent a right scrotal mass excision and orchiectomy and left subcapsular orchiectomy. Tissue from the right testis was strongly positive for prostatic specific acid phosphatase (PSAP) and NKX3.1, a prostatic tumor suppressor gene with high sensitivity and specificity for metastatic prostate adenocarcinoma. The final pathology report supported metastatic prostate adenocarcinoma (Fig. 1).
Figure 1:
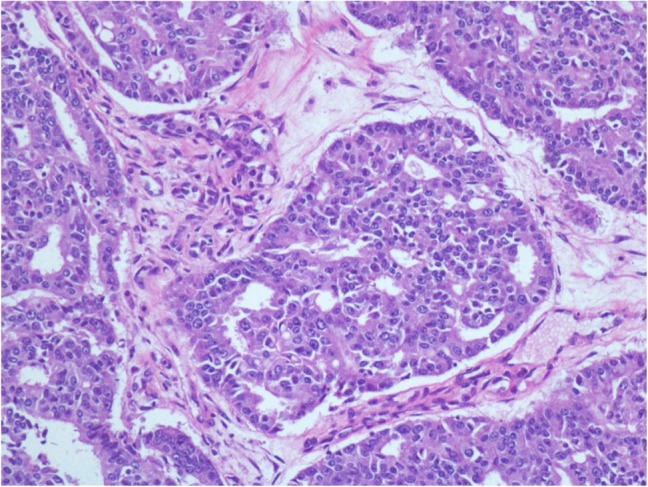
Right testicular mass showing infiltrating glands composed of cells with hyperchromatic, enlarged nuclei, prominent nucleoli and increased nuclear:cytoplasmic ratio arranged in cribriforming architecture.
Three months after his orchiectomy, the patient presented with altered mental status. The patient had recently moved to an assisted living facility due to an inability to perform activities of daily living in the setting of worsening memory loss and increasing delusions. His subacute decline in mental status was attributed to progression of underlying dementia.
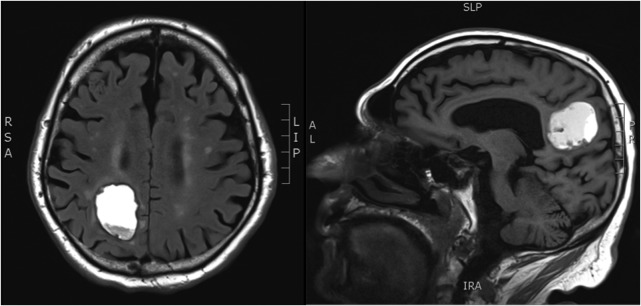
On examination, the patient was oriented only to self and scored a 13 out of 30 on the Saint Louis University Mental Status (SLUMS) examination. The remainder of his exam, which included a comprehensive neurological evaluation, was non-focal. Computed tomography (CT) of the head and magnetic resonance imaging (MRI) of the brain showed two parenchymal masses, the dominant one measuring 3.5 × 2.4 cm2 in the right parietal lobe with irregular walls raising suspicion for malignancy (Fig. 2).
Figure 2:
MRI brain demonstrating a mass lesion in the right parietal lobe that measures 3.5 × 2.4 cm2.
Given the patient’s history of metastatic pca, a serum PSA was measured at 0.39 ng/mL. CT chest, abdomen, and pelvis and a nuclear bone scan demonstrated no evidence of other metastases. The patient underwent biopsy of the right parietal lobe mass, which showed well-demarcated fragments of a neoplasm with glandular and papillary features (Fig. 3). Immunohistochemistry was weakly positive for PSA and strongly positive for NKX3.1 (Fig. 4). The final pathology report supported metastatic prostate adenocarcinoma.
Figure 3:
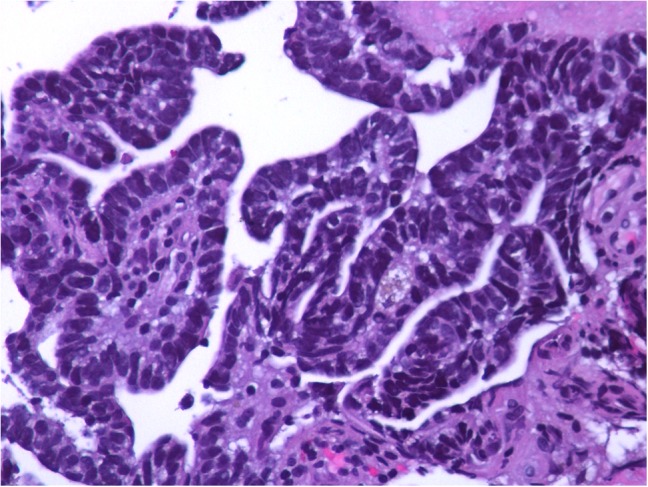
Biopsy of right parietal lobe mass showing well-demarcated fragments of a neoplasm with glandular and papillary features.
Figure 4:
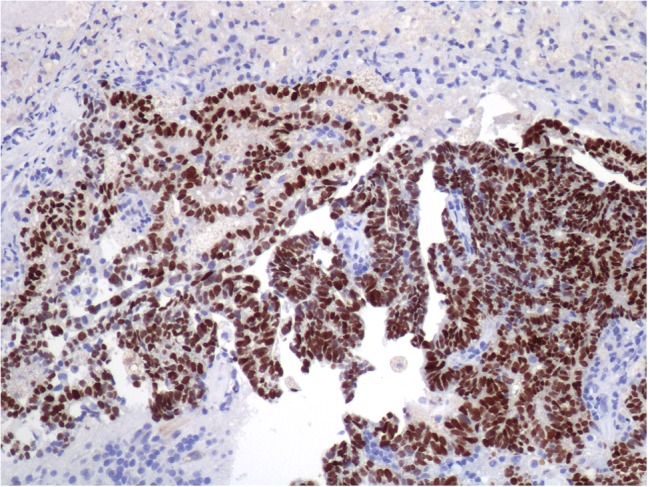
Biopsy of right parietal lobe mass with diffusely positive NKX3.1 stain.
The patient underwent treatment with dexamethasone 4 mg by mouth twice daily and five fractions of stereotactic brain radiotherapy. Hormonal manipulation was not recommended for adjuvant therapy since his serum testosterone was undetectable. After completing radiotherapy, he demonstrated a dramatic improvement in his memory and delusions. Six months after radiotherapy, the patient underwent repeat SLUMS examination and scored a 21 out of 30, demonstrating sustained improvement in his mental status.
DISCUSSION
This report describes a unique case in which a patient with previously treated prostate adenocarcinoma was found to have symptomatic metastases to two uncommon sites: the testis and brain.
The incidence of testicular neoplasms due to metastases of any origin is rare, occurring in 0.02–2.5% of patients [4]. The low incidence of testicular metastases may be secondary to lower temperatures in the scrotum leading to an unfavorable environment for tumor growth [5]. Although the tight junctions between Sertoli cells that comprise the blood-testis barrier can be altered as previously described in testicular carcinoma in situ [6], it is unclear why certain neoplasms might breach this barrier more easily. Clinical manifestations of testicular metastases, as seen in our patient, are also unusual, with most secondary neoplasms of the testis discovered incidentally in orchiectomy specimens or autopsy studies [4].
Brain metastasis from pca is also a rare phenomenon with a reported incidence of 0.63% [7]. The low frequency of CNS involvement suggests brain parenchyma is resistant to the establishment of metastatic foci by pca cells. While the presence of the blood-brain barrier may contribute to this resistance, metastatic breast cancer has been shown to compromise this barrier via increased vascular permeability [8], though there are no similar studies surrounding pca. Predominant symptoms of brain metastasis from pca include delirium (50%), headache (34%) and short-term memory deficits (17%) [7].
In one retrospective review, squamous cell carcinoma and cribriform subtypes were far more likely than adenocarcinoma to metastasize to the brain (relative risk, 20.36) [7]. In addition, <1% of patients with brain metastasis had no history of metastatic disease to common sites including bone, lung, liver or adrenals. Our patient had the unusual feature of adenocarcinoma metastasizing to the brain despite the lack of metastasis to more common locations.
The glandular and papillary features of our patient’s brain metastasis are consistent with a rare subtype of prostate adenocarcinoma referred to as ductal adenocarcinoma [9]. While the behavior of prostatic ductal adenocarcinoma is similar to that of the more common acinar adenocarcinoma, the ductal morphology predicts a more aggressive course. Though prostate adenocarcinoma metastasizes to the brain less frequently than other types of pca, our patient’s ductal subtype may have conferred increased risk for brain metastasis.
Serum PSA level is considered the most valuable tool for monitoring disease status in patients with advanced pca. One study noted that <1% of patients with metastatic pca present with ‘PSA-negative’ disease, defined as a serum PSA of <10 ng/mL [10]. This group tends to have high tumor grade (Gleason score 8–10) and poorer overall survival compared to those with PSA-positive metastatic pca. While our patient’s PSA was elevated during his workup for testicular metastasis, his serum PSA was negative when he was diagnosed with the intracranial mass.
To conclude, testicular and brain metastases from pca are extremely rare. Despite the potential for further spread from a testicular metastasis, more data is needed before recommending routine screening of the brain or other sites when a pca metastasis to the testis is diagnosed. Clinicians should be cautious not to exclude metastatic pca solely due to unusual location, lack of widely disseminated disease or negative PSA.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2017;67:7. [DOI] [PubMed] [Google Scholar]
- 2. Humphrey PA. Cancers of the male reproductive organs In: Stewart BW, Wild CP, eds.. World Cancer Report. Lyon: World Health Organization, 2014. [Google Scholar]
- 3. Craig J, Woulfe J, Sinclair J, Malone S. Isolated brain metastases as first site of recurrence in prostate cancer: case report and review of the literature. Curr Oncol 2015;22:e493–7. Case Report CurrOncology 2015.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dutt N, Bates AW, Baithun SI. Secondary neoplasms of the male genital tract with different patterns of involvement in adults and children. Histopathology 2000;37:323–31. [DOI] [PubMed] [Google Scholar]
- 5. Smallman LA, Odedra JK. Primary carcinoma of sigmoid colon metastasizing to epididymis. Urology 1984;23:598–9. [DOI] [PubMed] [Google Scholar]
- 6. Fink C, Weigel R, Hembes T, Lauke Wettwer H, Kliesch S, Bergmann M, et al. Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in situ. Neoplasia 2006;8:1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tremont-Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, Puduvalli VK. Brain metastasis from prostate carcinoma: The M.D. Anderson Cancer Center experience. Cancer 2003;98:363–8. [DOI] [PubMed] [Google Scholar]
- 8. Mittapalli RK, Adkins CE, Bohn KA, Mohammad AS, Lockman JA, Lockman PR. Quantitative fluorescence microscopy measures vascular pore size in primary and metastatic brain tumors. Cancer Res 2017;77:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan C. Prostatic ductal adenocarcinoma. Urol Sci 2012;23:87–8. [Google Scholar]
- 10. Birtle AJ, Freeman A, Masters JR, Payne HA, Harland SJ. BAUS Section of Oncology Cancer Registry . Clinical features of patients who present with metastatic prostate carcinoma and serum prostate-specific antigen (PSA) levels < 10 ng/mL: the ‘PSA negative’ patients. Cancer 2003;98:2362–7. [DOI] [PubMed] [Google Scholar]