Abstract
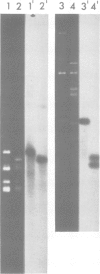
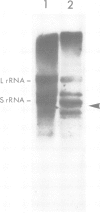
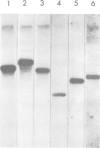
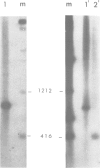
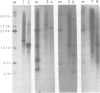
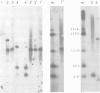
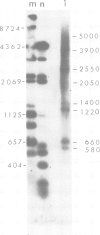
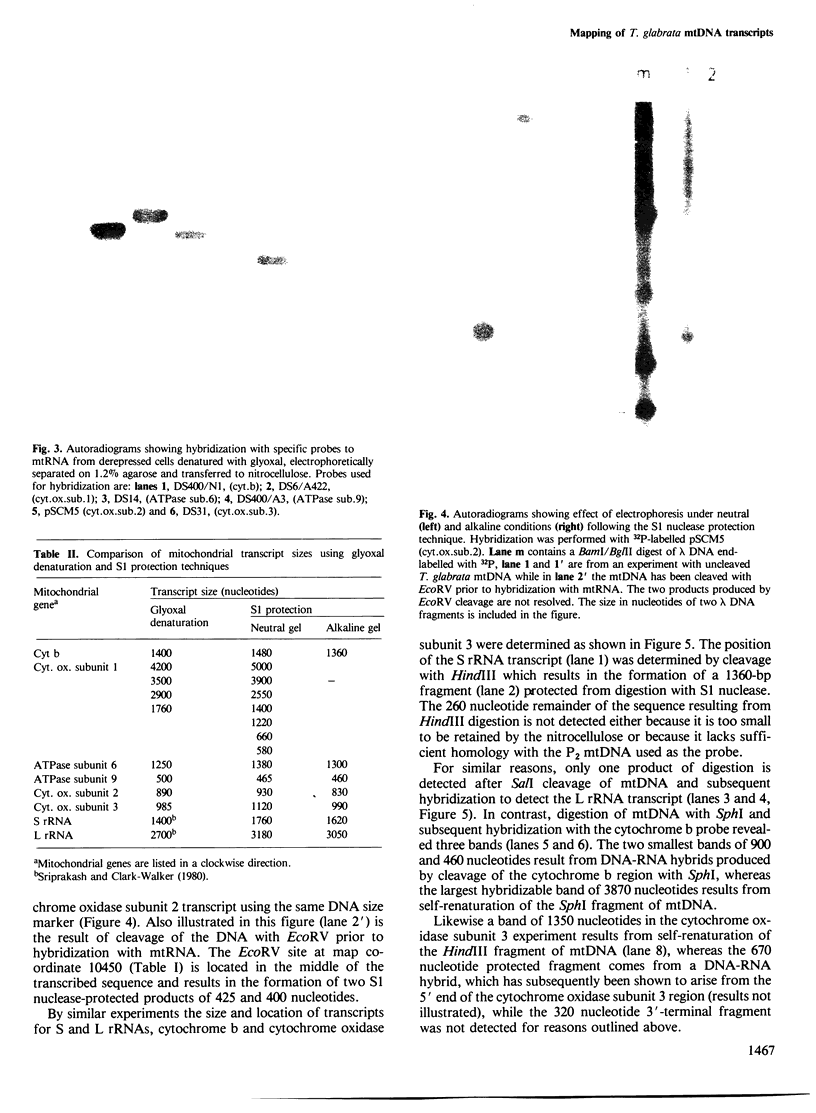
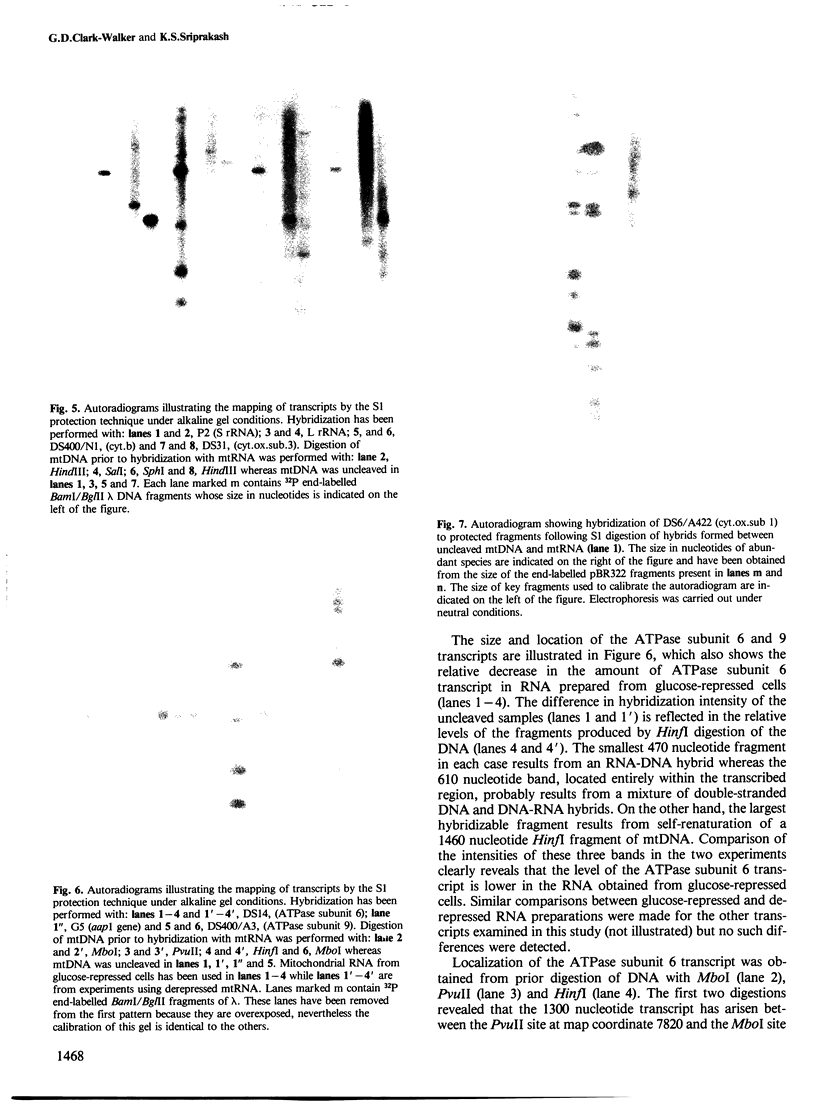
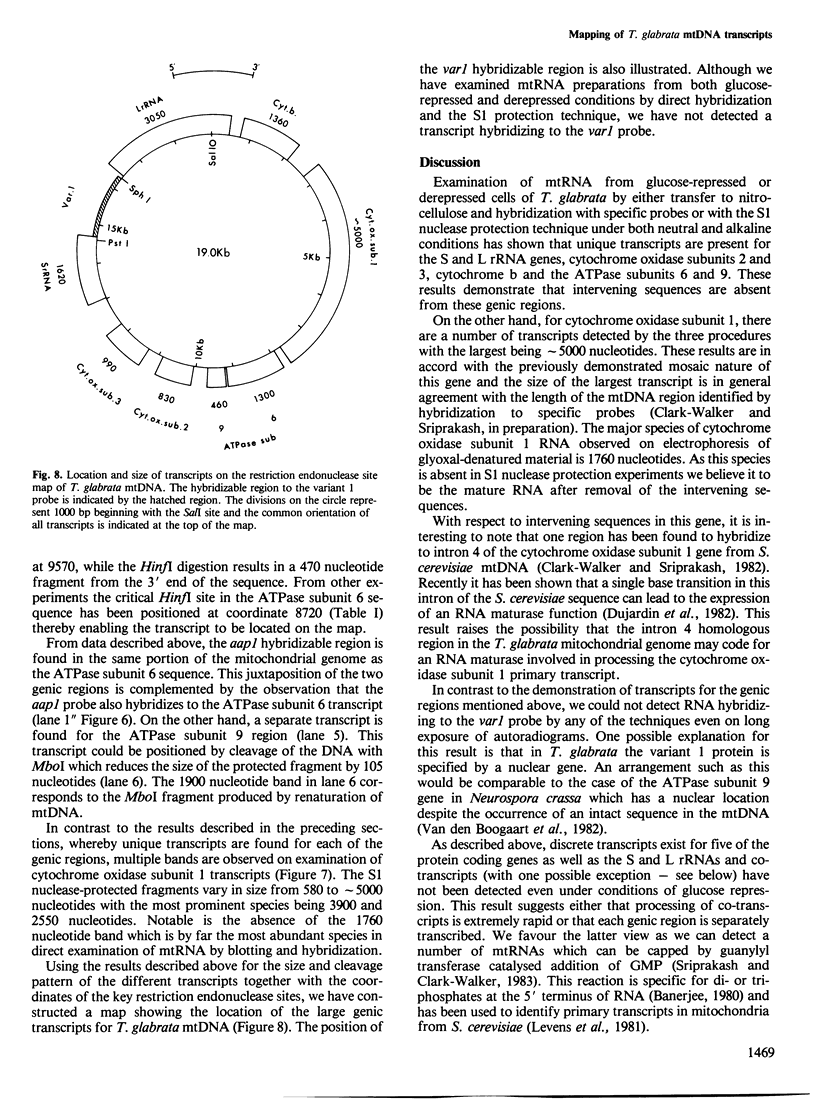
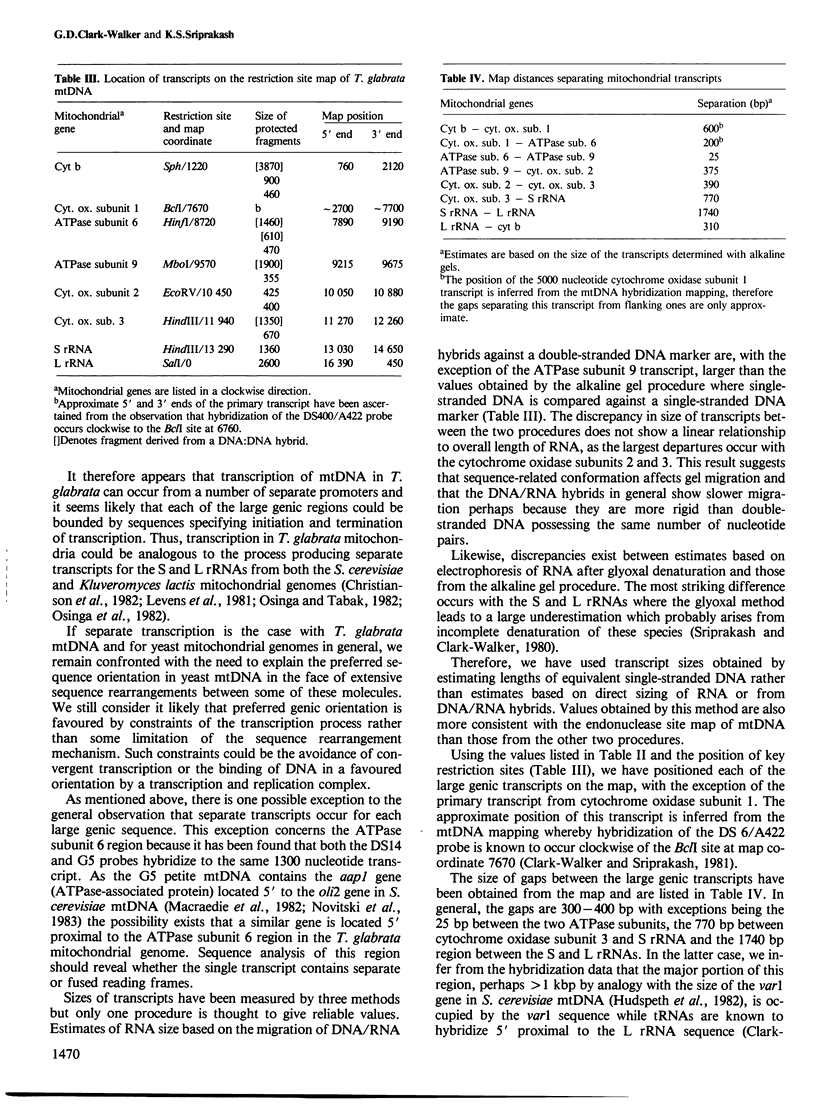
Unique transcripts for cytochrome b, ATPase subunits 6 and 9, cytochrome oxidase subunits 2 and 3 and S and L rRNA have been mapped by the S1 protection technique to the circular 19-kbp mitochondrial DNA (mtDNA) of the yeast Torulopsis glabrata. In contrast, a number of transcripts have been detected for the mosaic cytochrome oxidase subunit 1 gene with the largest being approximately 5000 nucleotides and the mature message having a length of 1760 nucleotides. Despite the presence in T. glabrata mtDNA of a sequence that hybridizes to the variant 1 gene of Saccharomyces cerevisiae mtDNA we have not detected a transcript of this region. Neither have we detected co-transcripts of adjacent genes in RNA from either glucose-repressed or derepressed cells. However, by comparison of RNA species from the two growth conditions, we have found that the ATPase subunit 6 transcript is lower in amount relative to other species in preparations from glucose-repressed cells. This information, together with the observation of separate transcripts and the knowledge that there are several species of mitochondrial RNA which can be capped by the guanylyl transferase catalysed addition of GMP, suggests that each of the genes investigated in the present study is separately transcribed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Edwards J., Levens D., Locker J., Rabinowitz M. Transcriptional initiation and processing of the small ribosomal RNA of yeast mitochondria. J Biol Chem. 1982 Jun 10;257(11):6494–6500. [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Sriprakash K. S. Sequence rearrangements between mitochondrial DNAs of Torulopsis glabrata and Kloeckera africana identified by hybridization with six polypeptide encoding regions from Saccharomyces cerevisiae mitochondrial DNA. J Mol Biol. 1981 Sep 25;151(3):367–387. doi: 10.1016/0022-2836(81)90002-4. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B., Langman L., Ash G., Becker M., Gibson F. Three genes coding for subunits of the membrane sector (F0) of the Escherichia coli adenosine triphosphatase complex. J Bacteriol. 1981 Jan;145(1):200–210. doi: 10.1128/jb.145.1.200-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin G., Jacq C., Slonimski P. P. Single base substitution in an intron of oxidase gene compensates splicing defects of the cytochrome b gene. Nature. 1982 Aug 12;298(5875):628–632. doi: 10.1038/298628a0. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth M. E., Ainley W. M., Shumard D. S., Butow R. A., Grossman L. I. Location and structure of the var1 gene on yeast mitochondrial DNA: nucleotide sequence of the 40.0 allele. Cell. 1982 Sep;30(2):617–626. doi: 10.1016/0092-8674(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Levens D., Ticho B., Ackerman E., Rabinowitz M. Transcriptional initiation and 5' termini of yeast mitochondrial RNA. J Biol Chem. 1981 May 25;256(10):5226–5232. [PubMed] [Google Scholar]
- Li M., Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979 Sep;18(1):47–53. doi: 10.1016/0092-8674(79)90352-0. [DOI] [PubMed] [Google Scholar]
- McArthur A. J., Monteith J. L. Air movement and heat loss from sheep. II. Thermal insulation of fleece in wind. Proc R Soc Lond B Biol Sci. 1980 Aug 13;209(1175):209–217. doi: 10.1098/rspb.1980.0091. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J., Christianson T., Levens D., Rabinowitz M., Attardi G. Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7195–7199. doi: 10.1073/pnas.79.23.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley K. M., Clark-Walker G. D. Abnormal mitochondrial genomes in yeast restored to respiratory competence. Genetics. 1978 Nov;90(3):517–530. doi: 10.1093/genetics/90.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., De Haan M., Christianson T., Tabak H. F. A nonanucleotide sequence involved in promotion of ribosomal RNA synthesis and RNA priming of DNA replication in yeast mitochondria. Nucleic Acids Res. 1982 Dec 20;10(24):7993–8006. doi: 10.1093/nar/10.24.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Tabak H. F. Initiation of transcription of genes for mitochondrial ribosomal RNA in yeast: comparison of the nucleotide sequence around the 5'-ends of both genes reveals a homologous stretch of 17 nucleotides. Nucleic Acids Res. 1982 Jun 25;10(12):3617–3626. doi: 10.1093/nar/10.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G. Electron microscopic visualization of mitochondrial RNA-DNA hybrids. J Mol Biol. 1971 Jan 28;55(2):267–270. doi: 10.1016/0022-2836(71)90196-3. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Séquence nucléotidique du gène de l'ARN ribosomique 15S mitochondrial de la levure. C R Seances Acad Sci D. 1980 Dec 8;291(12):933–936. [PubMed] [Google Scholar]
- Sriprakash K. S., Clark-Walker G. D. The size of yeast mitochondrial ribosomal RNAs. Biochem Biophys Res Commun. 1980 Mar 13;93(1):186–193. doi: 10.1016/s0006-291x(80)80264-6. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalenfeld B. E., Hill J., Tzagoloff A. Assembly of the mitochondrial membrane system. Characterization of the oxi2 transcript and localization of its promoter in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1983 Jan 10;258(1):610–615. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaart P., Samallo J., Agsteribbe E. Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature. 1982 Jul 8;298(5870):187–189. doi: 10.1038/298187a0. [DOI] [PubMed] [Google Scholar]