Abstract
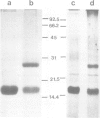
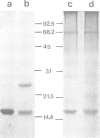
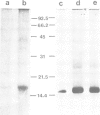
A new method for the purification of gap junctions is described which depends on the extraction of cell monolayers or tissue homogenates with Triton X-100. The major band on SDS-polyacrylamide gel electrophoresis (PAGE) of junctional preparations from a variety of vertebrate sources has an apparent mol. wt. of 16,000 (16 K). Further evidence for the junctional origin of the 16 K protein is provided by the results of four different experimental approaches. (i) The junctions form a sharp band in potassium iodide density gradients at 1.195 g/cm3 and the 16 K protein is the only detectable band in fractions of this bouyant density. (ii) The junctions are progressively solubilised by increasing concentrations of SDS (in the range 0.1-0.5%) and the dissolution of the junctional structure, observed by electron microscopy, parallels the release of the 16 K protein. (iii) Glutaraldehyde fixation of intact junctions cross-links the 16 K protein. (iv) The recoverable amount of the 16 K protein correlates with known changes in gap junctional area in the regenerating weanling rat liver after partial hepatectomy and in V79 cell cultures exposed to 4beta-phorbol 12-myristate 13-acetate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bok D., Dockstader J., Horwitz J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol. 1982 Jan;92(1):213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. F., Goodenough D. A. Five-hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981 Aug;90(2):521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M. E., Pitts J. D. Permeability of junctions between animal cells. Intercellular exchange of various metabolites and a vitamin-derived cofactor. Exp Cell Res. 1981 Jan;131(1):1–13. doi: 10.1016/0014-4827(81)90399-2. [DOI] [PubMed] [Google Scholar]
- Finbow M., Yancey S. B., Johnson R., Revel J. P. Independent lines of evidence suggesting a major gap junctional protein with a molecular weight of 26,000. Proc Natl Acad Sci U S A. 1980 Feb;77(2):970–974. doi: 10.1073/pnas.77.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg-Newton J., Simpson I., Loewenstein W. R. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979 Jul 27;205(4404):404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J Cell Biol. 1974 May;61(2):557–563. doi: 10.1083/jcb.61.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A. In vitro formation of gap junction vesicles. J Cell Biol. 1976 Feb;68(2):220–231. doi: 10.1083/jcb.68.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972 Sep;54(3):646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D., Eibl H., Weber K. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol. 1979 Aug 5;132(2):193–218. doi: 10.1016/0022-2836(79)90391-7. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979 Mar 25;254(6):2138–2147. [PubMed] [Google Scholar]
- Janssen-Timmen U., Dermietzel R., Frixen U., Leibstein A., Traub O., Willecke K. Immunocytochemical localization of the gap junction 26 K protein in mouse liver plasma membranes. EMBO J. 1983;2(3):295–302. doi: 10.1002/j.1460-2075.1983.tb01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Goodenough D. A. Isolation of mouse myocardial gap junctions. J Cell Biol. 1980 Sep;86(3):755–764. doi: 10.1083/jcb.86.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Amos J. Inhibition of metabolic cooperation between mammalian cells in culture by tumor promoters. Carcinogenesis. 1981;2(3):243–249. doi: 10.1093/carcin/2.3.243. [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Hunkapiller M. W., Grim L. B., Hood L. E., Revel J. P. Rat liver gap junction protein: properties and partial sequence. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7594–7598. doi: 10.1073/pnas.78.12.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L., Goodenough D. A. Preparation, characterization, and localization of antisera against bovine MP26, an integral protein from lens fiber plasma membrane. J Cell Biol. 1983 Mar;96(3):625–632. doi: 10.1083/jcb.96.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. Low resistance junctions in crayfish. I. Two arrays of globules in junctional membranes. J Cell Biol. 1973 Apr;57(1):66–76. doi: 10.1083/jcb.57.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. Low resistance junctions in crayfish. II. Structural details and further evidence for intercellular channels by freeze-fracture and negative staining. J Cell Biol. 1973 Apr;57(1):54–65. doi: 10.1083/jcb.57.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts J. D., Burk R. R. Specificity of junctional communication between animal cells. Nature. 1976 Dec 23;264(5588):762–764. doi: 10.1038/264762a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Simms J. W. Permeability of junctions between animal cells. Intercellular transfer of nucleotides but not of macromolecules. Exp Cell Res. 1977 Jan;104(1):153–163. doi: 10.1016/0014-4827(77)90078-7. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Yee A. G., Hudspeth A. J. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2924–2927. doi: 10.1073/pnas.68.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. D. Membrane structure. J Cell Biol. 1981 Dec;91(3 Pt 2):189s–204s. doi: 10.1083/jcb.91.3.189s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., Easter D., Revel J. P. Cytological changes in gap junctions during liver regeneration. J Ultrastruct Res. 1979 Jun;67(3):229–242. doi: 10.1016/s0022-5320(79)80024-6. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., Edens J. E., Trosko J. E., Chang C. C., Revel J. P. Decreased incidence of gap junctions between Chinese hamster V-79 cells upon exposure to the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate. Exp Cell Res. 1982 Jun;139(2):329–340. doi: 10.1016/0014-4827(82)90257-9. [DOI] [PubMed] [Google Scholar]