Abstract
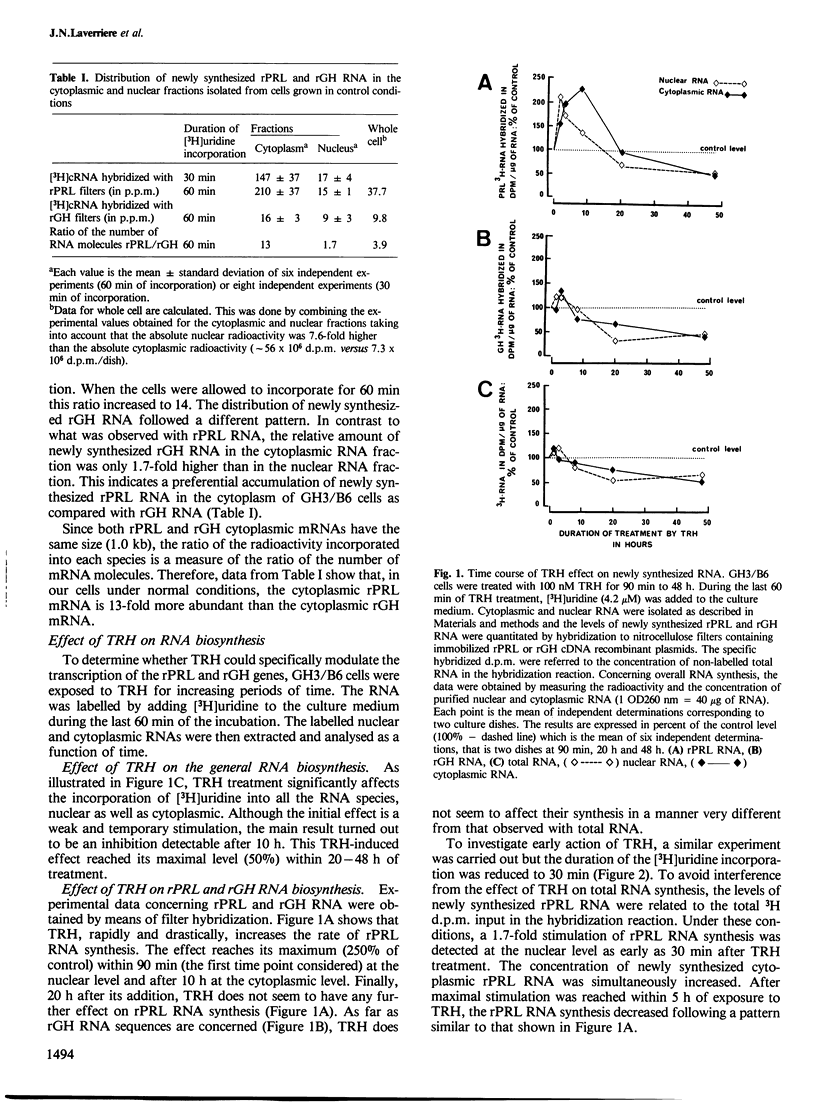
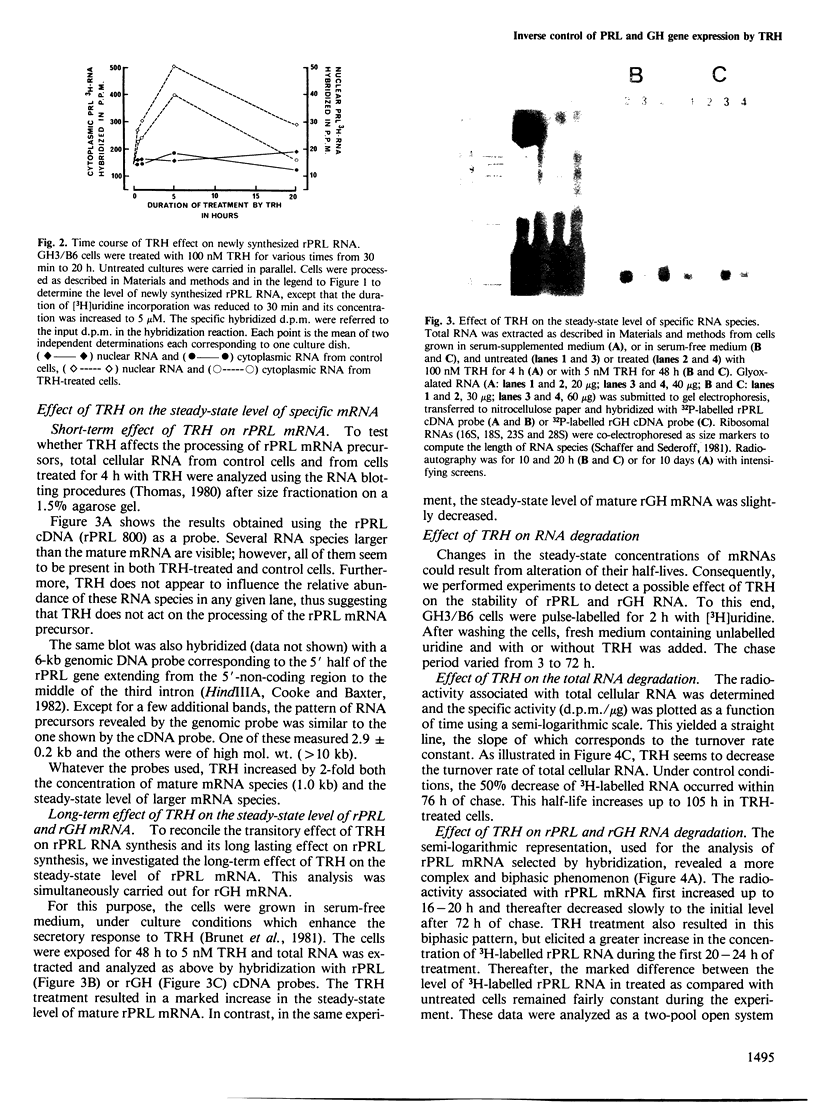
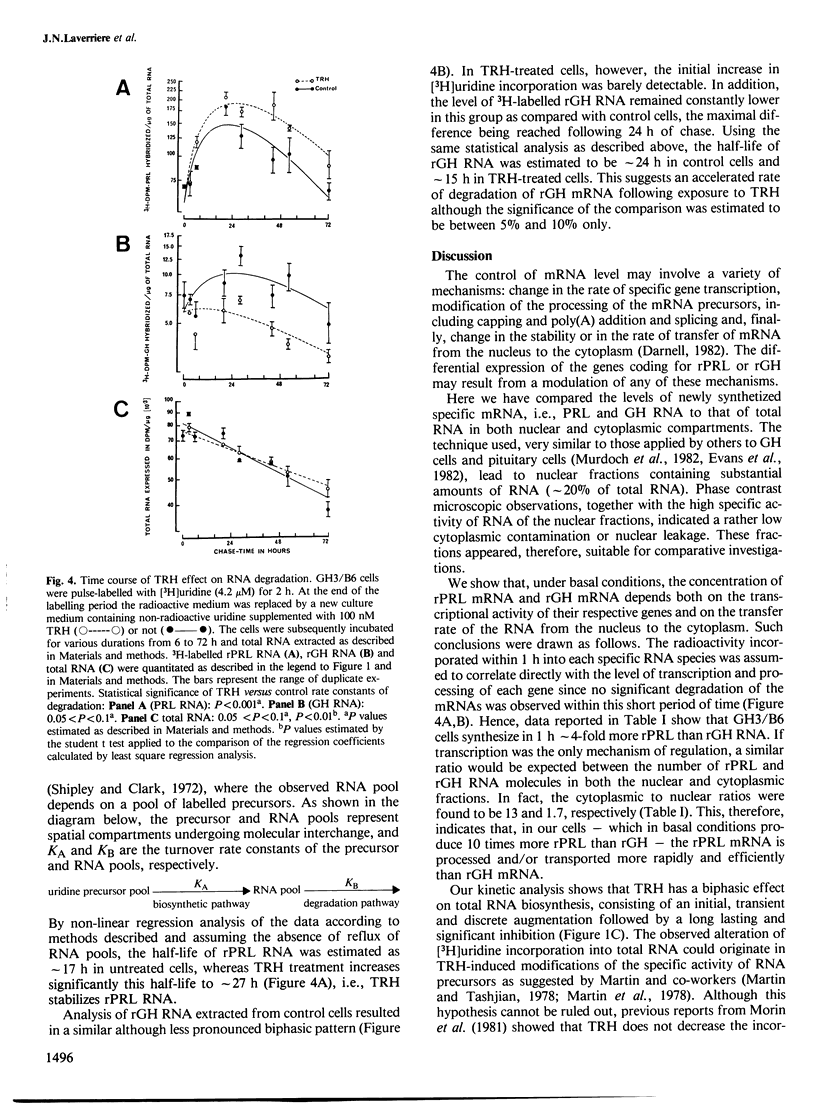
The hypothalamic tripeptide thyroliberin (TRH) regulates prolactin (PRL) and growth hormone (GH) synthesis inversely by modulating the levels of their specific mRNA. Changes in mRNA levels could involve both transcriptional and posttranscriptional events. To examine further these possibilities, we have investigated the effect of TRH on the biosynthesis and degradation of PRL and GH RNA in a rat pituitary tumor cell line. Newly synthesized PRL and GH RNA sequences were quantified in nuclear and cytoplasmic fractions by hybridization of 3H-labelled RNA to immobilized plasmid DNA containing either PRL or GH cDNA sequences. Steady-state levels of specific RNA were estimated by RNA blot hybridization. The results indicate that TRH increases in a rapid but transient manner the transcription of the PRL gene, and suggest that it does not alter the processing and the transport to the cytoplasm. In contrast, after a lag-time, TRH seems to induce a long-lasting inhibition on GH, as well as on overall gene transcription. Furthermore, we observed an effect of TRH on mRNA stability. TRH significantly increases the half-life of PRL mRNA. Our results also support the hypothesis that TRH decreases the half-life of GH mRNA. Such post-transcriptional action of TRH amplifies and prolongs the regulations exerted at the transcriptional level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barta A., Richards R. I., Baxter J. D., Shine J. Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4867–4871. doi: 10.1073/pnas.78.8.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K., Gräf K. J., Faivre-Bauman A., Beier S., Tixier-Vidal A., Kleinkauf H. Inhibition of prolactin secretion by histidyl-proline-diketopiperazine. Nature. 1978 Jul 13;274(5667):174–175. doi: 10.1038/274174a0. [DOI] [PubMed] [Google Scholar]
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Biswas D. K., Hanes S. D., Brennessel B. A. Mechanism of induction of prolactin synthesis in GH cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):66–70. doi: 10.1073/pnas.79.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournaud F., Gourdji D., Mongongu S., Tixier-Vidal A. [3H]-Thyroliberin (TRH) binding to nuclei isolated from a pituitary clonal cell line (GH3). Neuroendocrinology. 1977;24(3-4):183–194. doi: 10.1159/000122760. [DOI] [PubMed] [Google Scholar]
- Brunet N., Rizzino A., Gourdji D., Tixier-Vidal A. Effects of thyroliberin (TRH) on cell proliferation and prolactin secretion by GH3/B6 rat pituitary cells: a comparison between serum-free and serum-supplemented media. J Cell Physiol. 1981 Nov;109(2):363–372. doi: 10.1002/jcp.1041090220. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Baxter J. D. Structural analysis of the prolactin gene suggests a separate origin for its 5' end. Nature. 1982 Jun 17;297(5867):603–606. doi: 10.1038/297603a0. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Shine J., Baxter J. D., Martial J. A. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981 Apr 25;256(8):4007–4016. [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Weiner R. I., Baxter J. D., Martial J. A. Structure of cloned DNA complementary to rat prolactin messenger RNA. J Biol Chem. 1980 Jul 10;255(13):6502–6510. [PubMed] [Google Scholar]
- Dannies P. S., Tashjian A. H., Jr Thyrotropin-releasing hormone increases prolactin mRNA activity in the cytoplasm of GH-cells as measured by translation in a wheat germ cell-free system. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1180–1189. doi: 10.1016/0006-291x(76)91027-5. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., David D. N., Rosenfeld M. G. Regulation of prolactin and somatotropin mRNAs by thyroliberin. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1294–1298. doi: 10.1073/pnas.75.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Rosenfeld M. G. Cell-free synthesis of a prolactin precursor directed by mRNA from cultured rat pituitary cells. J Biol Chem. 1976 May 10;251(9):2842–2847. [PubMed] [Google Scholar]
- Evans R. M., Birnberg N. C., Rosenfeld M. G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Thyrotropin-releasing hormone regulates the number of its own receptors in the GH3 strain of pituitary cells in culture. Biochemistry. 1975 Aug 26;14(17):3845–3851. doi: 10.1021/bi00688a017. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Fritsch M. K., Gorski J. Probable nuclear precursors of preprolactin mRNA in rat pituitary cells. J Biol Chem. 1981 Mar 25;256(6):2597–2600. [PubMed] [Google Scholar]
- Laverriere J. N., Gourdji D., Picart R., Tixier-Vidal A. Thyroliberin is rapidly transferred to the nucleus of GH3 pituitary cells at both 4 degree C and 37 degree C. Biochem Biophys Res Commun. 1981 Dec 15;103(3):833–840. doi: 10.1016/0006-291x(81)90886-x. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Cort A. M., Tashjian A. H., Jr Thyrotropin-releasing hormone modulation of uridine uptake in rat pituitary cells. Characterization of the responses. J Biol Chem. 1978 Jan 10;253(1):99–105. [PubMed] [Google Scholar]
- Martin T. F., Tashjian A. H., Jr Thyrotropin-releasing hormone modulation of uridine uptake in rat pituitary cells. Evidence that uridine phosphorylation is regulated. J Biol Chem. 1978 Jan 10;253(1):106–115. [PubMed] [Google Scholar]
- Maurer R. A. Estradiol regulates the transcription of the prolactin gene. J Biol Chem. 1982 Mar 10;257(5):2133–2136. [PubMed] [Google Scholar]
- Maurer R. A., Gubbins E. J., Erwin C. R., Donelson J. E. Comparison of potential nuclear precursors for prolactin and growth hormone messenger RNA. J Biol Chem. 1980 Mar 25;255(6):2243–2246. [PubMed] [Google Scholar]
- Maurer R. A. Thyroid hormone specifically inhibits prolactin synthesis and decreases prolactin messenger ribonucleic acid levels in cultured pituitary cells. Endocrinology. 1982 May;110(5):1507–1514. doi: 10.1210/endo-110-5-1507. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A., Auffray C., Tixier-Vidal A. Effect of thyroliberin on the biosynthesis of poly(A)-mRNA species in GH3 cells. Direct evidences for alterations in their distribution profiles and increased synthesis of the preprolactin mRNA. FEBS Lett. 1981 Aug 17;131(1):122–126. doi: 10.1016/0014-5793(81)80902-7. [DOI] [PubMed] [Google Scholar]
- Murdoch G. H., Potter E., Nicolaisen A. K., Evans R. M., Rosenfeld M. G. Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature. 1982 Nov 11;300(5888):192–194. doi: 10.1038/300192a0. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Hogan M. L., Sauer R., Rosenblum I. Y., Greenwood F. C. Sequences of pituitary and placental lactogenic and growth hormones: evolution from a primordial peptide by gene reduplication. Proc Natl Acad Sci U S A. 1971 Apr;68(4):866–870. doi: 10.1073/pnas.68.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E., Nicolaisen A. K., Ong E. S., Evans R. M., Rosenfeld M. G. Thyrotropin-releasing hormone exerts rapid nuclear effects to increase production of the primary prolactin mRNA transcript. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6662–6666. doi: 10.1073/pnas.78.11.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C., Wilber J. F., Akerstrom V., Banerji A. Cyclo (His-Pro): a selective inhibitor of rat prolactin secretion in vitro. Life Sci. 1980 Nov 24;27(21):1979–1983. doi: 10.1016/0024-3205(80)90418-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ronning S. A., Heatley G. A., Martin T. F. Thyrotropin-releasing hormone mobilizes Ca2+ from endoplasmic reticulum and mitochondria of GH3 pituitary cells: characterization of cellular Ca2+ pools by a method based on digitonin permeabilization. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6294–6298. doi: 10.1073/pnas.79.20.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer H. E., Sederoff R. R. Improved estimation of DNA fragment lengths from Agarose gels. Anal Biochem. 1981 Jul 15;115(1):113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Receptor-mediated release of plasma membrane-associated calcium and stimulation of calcium uptake by thyrotropin-releasing hormone in pituitary cells in culture. J Biol Chem. 1981 Sep 10;256(17):8994–9002. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Bancroft F. C., Levine L. Production of both prolactin and growth hormone by clonal strains of rat pituitary tumor cells. Differential effects of hydrocortisone and tissue extracts. J Cell Biol. 1970 Oct;47(1):61–70. doi: 10.1083/jcb.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr Clonal strains of hormone-producing pituitary cells. Methods Enzymol. 1979;58:527–535. doi: 10.1016/s0076-6879(79)58167-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegnez M., Schachter B. S., Baxter J. D., Martial J. A. Hormonal regulation of growth hormone mRNA. DNA. 1982;1(2):145–153. doi: 10.1089/dna.1.1982.1.145. [DOI] [PubMed] [Google Scholar]
- White B. A., Bauerle L. R., Bancroft F. C. Calcium specifically stimulates prolactin synthesis and messenger RNA sequences in GH3 cells. J Biol Chem. 1981 Jun 25;256(12):5942–5945. [PubMed] [Google Scholar]