Abstract
Background
Ukraine’s volatile syndemics of tuberculosis (TB) and HIV among people who inject drugs (PWIDs) introduces numerous treatment challenges for each condition, including high mortality and development of multi-drug resistant TB (MDR-TB).
Methods
A prospective, non-randomized 90-day observational study was conducted in six Ukrainian TB treatment sites to assess the effectiveness of integrating methadone maintenance (MMT) with TB treatment using: (1) 90-day TB treatment retention; (2) time to treatment discontinuation; (3) TB medication adherence; and (4) subject disposition, including mortality. Of the 110 participants enrolled, 57 received MMT and 53 did not (non-MMT).
Results
All of the primary outcomes were significantly better in MMT versus non-MMT groups, including 90-day TB treatment completion (89.5% versus 73.6%; p = 0.031), time to TB treatment discontinuation (p = 0.039) and TB medication adherence (97.1% versus 86.2%; p<0.001) after controlling for death. The major reasons for treatment non-completion in the non-MMT group included death (N=3), administrative discharge from the clinic (N =5), loss to follow-up (N =2), and arrest (N=4). Overall, 90-day mortality was high (8.2%). After controlling for covariates differing between the two groups at baseline, the only independent predictor of completing 90 days of TB treatment was receipt of MMT in an integrated treatment setting (AOR = 3.05; 95% CI 1.08–8.66).
Conclusions
MMT integrated into inpatient TB treatment significantly improves retention in TB treatment and TB medication adherence among PWIDs. These findings call for policy change to increase the number of MMT sites in TB facilities and make MMT a low-threshold treatment option for opioid dependence in Ukraine.
Keywords: Methadone maintenance, Tuberculosis, HIV/AIDS, MDR-TB, Treatment retention, Adherence, Substance Abuse, Opioid dependence, Ukraine
Background
With nearly 2 billion people infected worldwide (Glaziou, Floyd, & Raviglione, 2009), tuberculosis (TB) remains a major global public health problem and contributes significantly to morbidity and mortality. In 2010, there were 8.8 million incident TB cases, of which there were 1.1 million TB-related deaths among people not infected with HIV. Co-infection with HIV and TB, however, creates challenges in both diagnosis and treatment, thus contributing to worse treatment outcomes and mortality rates exceeding 13%. Globally, the absolute annual number of incident TB cases has continued to decrease since 2006, with incidence decreasing by 1.3% annually since 2002. Though these findings are seen across all six global reporting regions, there has been little improvement in these findings in many countries of the former Soviet Union (FSU), including Eastern Europe and Central Asia – a region where both HIV and TB are high among drug using populations and MDR-TB is expanding (World Health Organization, 2011).
People who inject drugs (PWIDs) remain at high risk for tuberculosis (TB), including in low-, middle- and high-income countries. As a result, the twin epidemics of drug use and TB coexist in a number of countries globally. Treatment of TB among PWIDs poses a unique set of challenges for TB diagnosis and control efforts. In recognition of this special relationship the World Health Organization, the Joint United Nations Programme on HIV/AIDS, and the United Nations Office on Drugs and Crime, released guidelines to improve coordinated care for TB among PWIDs (World Health Organization, 2008). Among the recommendations is to integrate TB treatment with medication-assisted therapy for opioid dependence, and if indicated, with HIV treatment services (Sylla, Bruce, Kamarulzaman, & Altice, 2007; World Health Organization, 2008).
Ukraine, a country facing multiple challenges in the context of volatile epidemics of TB and HIV among PWIDs, faces a number of important challenges in optimizing treatment outcomes for each condition, including the development of multi-drug resistant TB (MDR-TB) (Dubrovina et al., 2008; Granich, 2008; Hurley, 2010; Kruglov et al., 2008; Kruk et al., 2011; Wolfe, Carrieri, & Shepard, 2010). Though Ukrainian MDR-TB rates are not fully known, one regional study conducted in 2006 identified a range from 15.5% to 41.5% among newly identified and previously treated TB cases in community settings, respectively (Dubrovina et al., 2008). HIV prevalence among PWIDs in Ukraine is estimated to be 21.5% (Ministry of Health of Ukraine, 2012), while TB prevalence data among PWIDs in Ukraine are currently not available, since no comprehensive assessment has been done. Nonetheless, PWIDs remain at high risk of developing active TB, especially in settings with high HIV and TB prevalence (Deiss, Rodwell, & Garfein, 2009; Getahun, Gunneberg, Sculier, Verster, & Raviglione, 2012). Officially reported TB case notification rate in Ukraine, likely an under-representation of the true epidemic, was 74 per 100,000 population in 2010 – more than twice the average rate for the European region that same year (World Health Organization, 2011). Though multiple factors contribute to the development of MDR-TB among PWIDs, poor adherence to therapy and discontinuation of treatment may all contribute to poor outcomes and such factors have been described as consequences of the lifestyle patterns of PWIDs, including the need to procure drugs on daily basis (Pablos-Mendez, Knirsch, Barr, Lerner, & Frieden, 1997; Story, Murad, Roberts, Verheyen, & Hayward, 2007).
In recognition that opioid dependence is a chronic, relapsing condition, this study compared the concomitant treatment of TB and opioid dependence using MMT, an evidence-based pharmacological treatment for opioid dependence, with TB patients not receiving MMT. To describe and understand better how to optimally organize TB treatment among PWIDs in Ukraine, the research compared 90-day treatment completion, time to TB treatment discontinuation, TB medication adherence and treatment disposition (including death) among hospitalized pulmonary TB patients with co-morbid opioid dependence being treated in an integrated care setting.
Methods
Study sites
A 90-day observational study was conducted in six TB treatment sites in Ukraine between December 2011 and April 2012. Directly observed therapy (DOT) is required for all TB patients in Ukraine according to the Ministry of Health guidelines. Unlike in other countries, the Ukrainian guidelines allow for TB patients who are deemed “unstable” (homeless, active drug users, previous treatment defaulters, etc.) or who have MDR-TB to be forcibly “hospitalized” for prolonged periods of time to reduce risk of transmission to others. This approach is the standard of care and common in most FSU countries. Medication acceptance and adherence, however, remains voluntary but is a condition for community release. As part of Ukraine’s new efforts to expand MMT throughout the country, it was made available in some TB hospital sites. MMT sites in Mykolayiv, Odesa and Kherson were selected because of the availability of comprehensive and integrated care sites that included the additional provision of MMT for hospitalized pulmonary TB patients. The comparison group was recruited from TB treatment hospitals in Kyiv, Donetsk, and Dnipropetrovsk where MMT was not provided within TB treatment settings. The standard of care includes consultation with a drug addiction specialist, if agreed by the TB doctor, and detoxification is prescribed by the doctor if indicated and agreed upon by the patient.
Study participants
A total of 110 participants were enrolled and prospectively followed for 90 days: N =57 in the study (MMT) group and N = 53 in the comparison (non-MMT) group. All study participants met the following inclusion criteria: (1) were ≥18 years; (2) met ICD-10 criteria for opioid dependence (Janca, Ustun, Early, & Sartorius, 1993); (3) had confirmed pulmonary TB diagnosis; (4) were prescribed at least 90 days of inpatient TB treatment from baseline enrolment; and (5) provided written informed consent. The fourth criterion was established in order to standardize outcome measurement, given that the TB status of patients may differ based on factors such as new or repeated treatment, different levels of underlying drug resistance, and duration and severity of TB disease which were not fully measured in this study.
Our final analytical sample compared those who were prescribed MMT with those who were not. No minimum MMT dose was required for study entry and dose varied considerably. All MMT was initiated at the time of pulmonary TB diagnosis in the integrated care site. Eight study participants in Odessa, Mykolayiv and Kherson, however, were ultimately analysed in the non-MMT group because they either refused MMT or did not meet legally established eligibility criteria for MMT enrolment in Ukraine (at least two previous documented unsuccessful treatment attempts using behavioural counselling). Assigning them to MMT would have incorrectly examined the impact of MMT (which they never received) and removing them from the analysis did not alter the final outcomes. All individuals enrolled in the study were assigned an anonymous code, which was linked with the data collection instruments. Participants were paid 80 UAH (~$10USD, 2012 exchange rate) for completion of interviews.
Data collection
Data were collected at baseline and throughout 90 days of observation. Baseline interviews included patient demographic and social characteristics, drug and alcohol use, psychiatric conditions, previous HIV testing and current status, prescription of antiretro-viral therapy (ART), previous TB treatment history and attitudes toward TB treatment (both groups) and MMT (MMT group only). Drug use portions were adapted from the Addiction Severity Index (ASI-lite), modified to include local Ukrainian drug use (McLellan, Luborsky, Woody, & O’Brien, 1980).
Chart reviews after 90 days recorded HIV testing, ART prescription, DOT record of taking observed TB and methadone medications, and reasons for treatment discontinuation (recorded by the TB doctor). Adverse side effects were not systematically recorded or obtained.
The TB treatment outcomes (dependent variables) included: (1) percentage of patients completing 90 days of TB treatment and continuing with treatment at the end of the study; (2) time to discontinuation of TB treatment; (3) percentage of dosage of TB medications taken over the 90 days of observation; and (4) disposition of study participants, including death and the reasons for drop-out. For the purpose of this study, treatment discontinuation was defined as not receiving TB medications for two weeks or more. For time-based measurements, the date of the last dose of TB medication taken was used for the date of treatment discontinuation.
Data analysis
Statistical analyses were performed using SPSS software for Windows (version 19.0, Chicago, IL). Characteristics of study subjects were mostly analysed as categorical variables, and collapsed where frequencies were small. Age, duration of drug use, number of TB medications prescribed, and number of days of hospitalization in the current treatment episode before the baseline assessment were dichotomized by their median values. The significance of difference between the MMT and non-MMT groups was analysed using Chi-square test or independent sample T-test as appropriate.
Kaplan–Meier analysis was performed for the two groups to depict time (number of days) to treatment discontinuation over 90 days of observation. Log rank (Mantel–Cox) test of equality of survival distributions was used to analyse the significance of survival difference between the MMT and non-MMT groups.
The bivariate and multivariate binary logistic regression analysis was conducted to test the association between the 90-day TB treatment completion prevalence and a number of independent covariates, including receiving MMT, and those baseline characteristics that were significantly different at p< 0.05 between the MMT and non-MMT groups. For the final multivariate regression model presented below, a p-value of 0.1 was used to enter a model and 0.05 to retain a covariate variable as significant. In all tables, the p-values are bolded if p < 0.05.
Ethical statement
All participants provided signed informed consent and were assigned a unique identifier. After 90 days of observation, the links between the unique identifier and the patient identifier were destroyed. The Institutional Review Board at the Ukrainian Institute on Public Health Policy approved the study.
Results
The baseline characteristics of the total study sample, MMT and non-MMT groups are presented in Table 1. The sample was mostly men (80.1%) in their mid-30s (median age = 36.0 years). The MMT group, compared to the non-MMT group, was significantly older (61.4% vs. 35.8% over 36 years old, p = 0.007), and had a longer average lifetime duration of drug use (64.9% vs. 32.1% had used drugs for more than 17 years, p = 0.001). The most commonly used opioid was liquid opioid prepared from acetylated poppy straw. A higher percentage of non-MMT study participants had been using amphetamines during the 30 days prior to hospitalization (24.5% vs. 5.3%, p = 0.004).
Table 1.
Characteristics of study participants (N =110).
| Characteristic | Total sample (N =110) | MMT(N=57) | No MMT(N=53) | p-value |
|---|---|---|---|---|
| Site | ||||
| Mykolayiv | 20 (18.2%) | 16 (28.1%) | 4 (7.5%) | <0.001 |
| Odessa | 25 (22.7%) | 22 (38.6%) | 3 (5.7%) | |
| Kherson | 20 (18.2%) | 19 (33.3%) | 1 (1.9%) | |
| Kyiv | 12 (10.9%) | 0 (0%) | 12 (22.6%) | |
| Donetsk | 13 (11.8%) | 0 (0%) | 13 (24.5%) | |
| Dnipropetrovsk | 20 (18.2%) | 0 (0%) | 20 (37.7%) | |
| Age (median = 36.0 years) | ||||
| ≤36 years old | 56 (50.9%) | 22 (38.6%) | 34 (64.2%) | 0.007 |
| >36 years old | 54 (49.1%) | 35 (61.4%) | 19 (35.8%) | |
| Gender | ||||
| Female | 21 (19.1%) | 10 (17.5%) | 11 (20.8%) | 0.669 |
| Male | 89 (80.9%) | 47 (82.5%) | 42 (79.2%) | |
| Educationa | ||||
| 5–9 years | 30 (27.3%) | 12 (21.1%) | 18 (34.0%) | 0.311 |
| 10–12 years | 38 (34.5%) | 21 (36.8%) | 17 (32.1%) | |
| Higher education or professional technical | 42 (38.2%) | 24 (42.1%) | 18 (34.0%) | |
| Marriedb | ||||
| Yes | 27 (24.5%) | 14 (24.6%) | 13 (24.5%) | 0.997 |
| No | 83 (75.5%) | 43 (75.4%) | 40 (75.5%) | |
| Employed when hospitalized | ||||
| Yes | 24 (21.8%) | 13 (22.8%) | 11 (20.8%) | 0.838 |
| No | 86 (78.2%) | 44 (77.2%) | 42 (79.2%) | |
| Income | ||||
| None | 35 (31.8%) | 19 (33.3%) | 16 (30.2%) | 0.723 |
| Any | 75 (68.2%) | 38 (66.7%) | 37 (69.8%) | |
| Living arrangements when hospitalized | ||||
| Alone | 17 (15.5%) | 8 (14.0%) | 9 (17.0%) | 0.747 |
| With partner | 31 (28.2%) | 15 (26.3%) | 16 (30.2%) | |
| With relatives/friends | 58 (52.7%) | 31 (54.4%) | 27 (50.9%) | |
| Homeless/lives in hospital | 4 (3.6%) | 3 (5.3%) | 1 (1.9%) | |
| Lifetime duration of drug use (median = 17.0 years) | ||||
| ≤ 17 years | 56 (50.9%) | 20 (35.1%) | 36 (67.9%) | 0.001 |
| >17 years | 54 (49.1%) | 37 (64.9%) | 17 (32.1%) | |
| Any lifetime amphetamines use | ||||
| Yes | 45 (40.9%) | 20 (35.1%) | 25 (47.2%) | 0.198 |
| No | 65 (59.1%) | 37 (64.9%) | 28 (52.8%) | |
| Amphetamines use 30 days prior to hospitalization | ||||
| Yes | 16 (14.5%) | 3 (5.3%) | 13 (24.5%) | 0.004 |
| No | 94 (85.5%) | 54 (94.7%) | 40 (75.5%) | |
| HIV status | ||||
| Positive | 75 (68.2%) | 39 (68.4%) | 36 (67.9%) | 0.679 |
| Negative | 15 (13.6%) | 9 (15.8%) | 6 (11.3%) | |
| Status unknown | 20 (18.2%) | 9 (15.8%) | 11 (20.8%) | |
| Ever received antiretroviral therapy (N=75) | ||||
| Yes | 50 (66.7%) | 23 (59.0%) | 27 (75.0%) | 0.141 |
| No | 25 (33.3%) | 16 (41.0%) | 9 (25.0%) | |
| History of incarcerations | ||||
| Yes | 94 (85.5%) | 50 (87.7%) | 44 (83.0%) | 0.485 |
| No | 16 (14.5%) | 7 (12.3%) | 9 (17.0%) | |
| Mean number of incarcerations (±SD), among those, who have ever been incarcerated | ||||
| (N = 94, Range: 1–12) | 2.87 ± 2.01 | 2.98 ± 2.18 | 2.75 ±1.82 | 0.583 |
| Tuberculosis treatment status | ||||
| New diagnosis | 21 (19.1%) | 10 (17.5%) | 11 (20.8%) | 0.669 |
| Previously treated | 89 (80.9%) | 47 (82.5%) | 42 (79.2%) | |
| Prescribed TB treatment | ||||
| First line (or unknown) | 69 (62.7%) | 38 (66.7%) | 31 (58.5%) | 0.376 |
| At least one second line medication | 41 (37.3%) | 19 (33.3%) | 22 (41.5%) | |
| Number of TB medications prescribed at baseline (median = 4 drugs, N = 108) | ||||
| <4 drugs (2–3 drugs) | 31 (28.7%) | 22 (38.6%) | 9 (17.6%) | 0.016 |
| ≥4 drugs (4–6 drugs) | 77 (71.3%) | 35 (61.4%) | 42 (82.4%) | |
| Mean number of TB medications prescribed at baseline (± SD), (Range: 2–6) | ||||
| 4.07 ± 1.13 | 3.82 ±1.17 | 4.35 ± 1.04 | 0.015 | |
| Duration of inpatient stay at the baseline date (median = 75.0 days) | ||||
| ≤75 days | 55 (50.0%) | 16 (28.1%) | 39 (73.6%) | <0.001 |
| >75 days | 55 (50.0%) | 41 (71.9%) | 14 (26.4%) | |
| Mean duration of inpatient stay at the baseline date | ||||
| (days, ±SD), (Range: 0–706) | 125.1 ±145.9 | 173.1 ±170.4 | 73.5 ±90.2 | <0.001 |
| Mean methadone dose | ||||
| (mg, ±SD), (Range: 12–225) | – | 81.2 ±40.0 | – | – |
Results are significant at the 0.05 level.
Indicated highest level of education attained.
Registered, unregistered marriage and having a partner; Unmarried, single, separated or widowed, including unknown status.
The mean baseline methadone dose in the MMT group was 81.2 ± 40.0mg (range: 12–225 mg). During the study period no methadone side effects were recorded, no changes in dosage prescribed, and MMT adherence among this group was 100% (data not shown). Patients on MMT generally assessed the quality of treatment positively: 52.6% were fully satisfied with the program, 45.6% were partially satisfied, and only 1.8% were dissatisfied. Nearly three quarters (73.7%) said that MMT was very helpful in reducing illegal drug use while 19.3% indicated it was somewhat helpful, and 7.0% reported it was not helpful at all (data not shown).
Of all participants, only 19.1% were newly diagnosed TB cases (similar between groups), with the remaining sample having been previously treated for TB (previous defaulters). Overall, 37.3% of the study participants were prescribed at least one second-line TB medication. It is unknown whether treatment regimens were tailored to drug resistance profile in this sample; however there were several cases with documented MDR-TB, for which isoniazid and/or rifampicin were prescribed. On average, the MMT group was prescribed significantly fewer TB medications (mean = 3.8 ±1.2 vs. 4.4 ±1.0 medications in the non-MMT group, p = 0.015) and had remained in the hospital longer than the comparison group (mean= 173.1 ± 170.4 days vs. 73.5 ±90.2 days, respectively, p < 0.001).
The prevalence of HIV in this sample was very high − 68.2% self-reported being HIV-infected or were tested HIV-positive during hospitalization, while the status of 18.2% of participants was unknown. Two thirds (66.7%) of patients known to be HIV-infected were prescribed ART.
All three primary outcomes differed significantly between the MMT and non-MMT groups (Fig. 1). The percentage of patients completing at least 90 days of treatment was higher in the MMT than non-MMT group (89.5% vs. 73.6%; p = 0.031). In the MMT group, the remaining 10.5% (N =6) of participants who did not complete 90 days of treatment died during the study period, and aside from death, retention was 100%. In the comparison group, however, 5.7% (N =3) patients died, while 20.7% (N =11) dropped out for the following reasons: arrested (N= 4), discharged from hospital due to violation of rules (N= 5), and lost to follow-up (N= 2). All hospital violations resulting in discharge involved use of alcohol or illegal drugs, and alleged violence towards clinic personnel in one case.
Fig. 1.
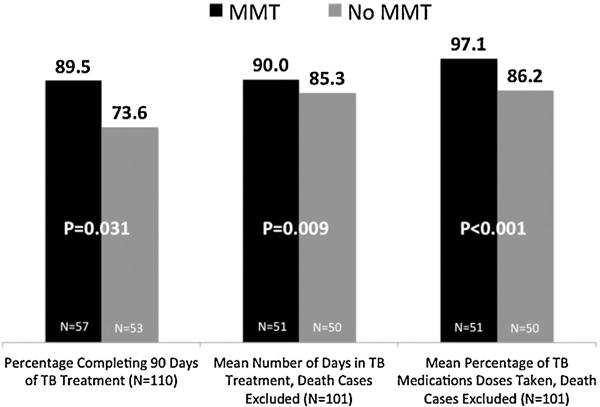
Tuberculosis treatment outcomes, stratified by receipt of methadone.
The mean number of days receiving TB treatment and mean percentage of prescribed TB medication dosages taken differed significantly between the two groups (Fig. 1, death cases excluded). Excluding death, MMT patients remained in treatment significantly, though modestly longer than non-MMT patients (90.0 ±0 vs. 85.3 ±12.1 days respectively; p = 0.009). Similarly, MMT patients had significantly higher adherence to prescribed TB medications than the non-MMT group (mean = 97.1% ±5.2% vs. 86.2% ±14.4% of TB medication doses taken; p< 0.001; death cases excluded). Even after excluding both mortality and drop-outs, adherence to prescribed TB medications still differed significantly between the two groups (mean = 97.1% ±5.2% vs. 91.3% ±7.2% of doses taken respectively, p< 0.001 (data not shown).
The time to discontinuation of TB treatment differed significantly between the two groups and is depicted in Fig. 2. Of note, retention in the non-MMT group dropped sharply after the 60th day of observation.
Fig. 2.
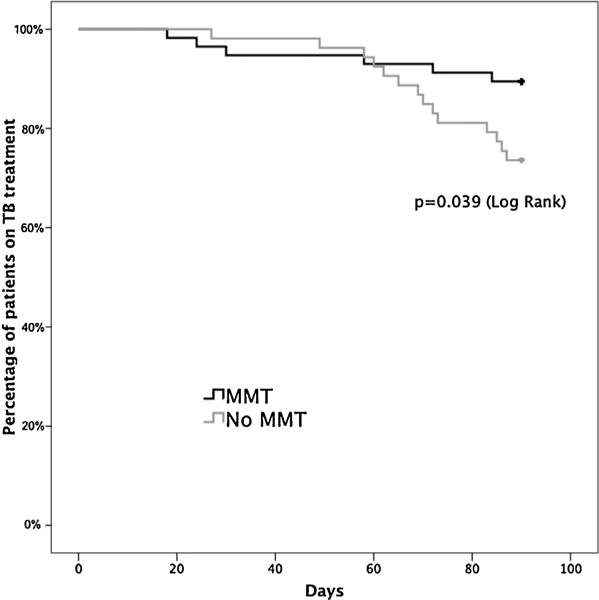
Time to discontinuation of TB treatment over 90-day observation period.
After controlling for all covariates significantly different at baseline, receipt of MMT was the only predictor significantly associated with 90-day TB treatment completion (Table 2) and while having used drugs for shorter duration approached significance for being negatively correlated with this outcome, it was collinear with receipt of MMT and affected the magnitude of the impact of MMT. These findings were robust irrespective of whether the 8 patients from MMT/TB settings who refused or were ineligible for MMT were assigned to the non-MMT group (data not shown).
Table 2.
Predictors of 90-day retention on tuberculosis treatment.
| Covariates | Bivariate association OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Methadone maintenance treatment | ||
| No | Referent | Referent |
| Yes | 3.05 (1.08–8.66) | 3.05 (1.08–8.66) |
| Site | ||
| Kherson | Referent | |
| Mykolayiv | 2.25 (0.36–13.97) | |
| Odessa | N/A(100% completion) | |
| Kyiv | 0.25 (0.05–1.21) | |
| Donetsk | 0.40 (0.08–1.91) | |
| Dnipropetrovsk | 1.42 (0.27–7.34) | |
| Age | ||
| >36 years old | Referent | |
| ≤36 years old | 0.64 (0.24–1.71) | |
| Lifetime duration of drug use (in years) | ||
| >17 years | Referent | |
| ≤17 years | 0.38 (0.13–1.06) | |
| Amphetamines use 30 days prior to hospitalization | ||
| Yes | Referent | |
| No | 1.63 (0.64–5.69) | |
| Number of tuberculosis medications prescribed at baseline | ||
| ≥4(4–6 drugs) | Referent | |
| <4 (2–3 drugs) | 1.36 (0.45–4.10) | |
| Duration of inpatient stay before the study observation period (days) | ||
| >75 days | Referent | |
| ≤75 days | 0.78 (0.30–2.07) | |
Results are significant at the 0.05 level.
Though there were no objective measures of improvement with regard to TB disease progression, the MMT group was significantly more likely than their counterparts to subjectively report that they believed their TB had improved after 90 days of treatment (80.4% vs. 62.4%; p = 0.036, data not shown).
Though not a primary outcome for this study, 90-day mortality was 8.2% (N = 9); of these patients, six were HIV-infected, one was HIV-negative, and two had unknown HIV status. None of HIV-infected patients who died during the course of the study was prescribed ART (Table 3). The cause of death was not documented. Moreover, though not a pre-determined outcome for this study, many patients, especially those who died, were receiving regimens deemed insufficient to treat their TB, especially for those with MDR-TB.
Table 3.
Selected characteristic of mortality cases among study participants.
| # | MMT | Site | Number of days in hospital before the study | Number of days in study before death | HIV-status | ART prescription | TB case status | Drug resistance status | Prescribed TB medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Mykolayiv | 101 | 72 | Negative | N/A | Previously treated | MDR-TB | H,R |
| 2 | Yes | Mykolayiv | 180 | 58 | Positive | No | Previously treated | MDR-TB | E, Z, Ox, Km, PAS, Pt |
| 3 | Yes | Kherson | 14 | 84 | Unknown | N/A | Previously treated | MDR-TBa | H,E,Z,Lx |
| 4 | Yes | Kherson | 429 | 18 | Positive | No | Previously treated | MDR-TBa | H, Z, PAS |
| 5 | Yes | Kherson | 45 | 24 | Positive | No | New diagnosis | Unknown | S, H, E, Z, Ox |
| 6 | Yes | Kherson | 470 | 30 | Unknown | N/A | Previously treated | MDR-TBa | H, Lx |
| 7 | No | Kyiv | 14 | 73 | Positive | No | Previously treated | MDR-TBa | H, R, Z, E, S |
| 8 | No | Donetsk | 0 | 58 | Positive | No | Previously treated | Unknown | H, R, Z, E, S |
| 9 | No | Dnipropetrovsk | 87 | 60 | Positive | No | Previously treated | MDR-TB | H, R, Z, E, Km |
MMT, methadone maintenance treatment; N/A, not applicable; ART, antiretroviral therapy; TB, tuberculosis; H, isoniazid; R, rifampicin; E, ethambutol; Z, pyrazinamide; Ox, of loxacin; Km, kanamycin; PAS, para-aminosalicylate sodium; Pt, protionamyde; Lx, levofloxacin; S, streptomycin.
Cinically suspected.
Discussion
Public health would dictate that successful TB treatment should be tailored to meet the needs of all patients with active disease. Thus, in settings like Ukraine where HIV, TB and opioid dependence are syndemic, successful TB treatment strategies to curtail its epidemic spread among PWIDs and the general community must simultaneously address all three conditions to optimize treatment outcomes. Failure to complete treatment for active TB and poor or intermittent compliance with all medications in multidrug regimens are two principal causes of treatment failure and the emergence of MDR-TB (Mahmoudi & Iseman, 1993). Such outcomes are especially concerning for PWIDs as HIV infection and crowded living conditions co-exist in congregate settings like prisons (reported by 85% of our sample). These are prevalent and magnify the risks associated with increased transmission and resistant disease.
To the best of our knowledge, this is the first study assessing the ‘real-world’ effectiveness of MMT in improving a number of TB treatment outcomes among PWIDs. This is especially crucial in Ukraine, a country with an emerging MDR-TB epidemic and where HIV and opioid dependence are common co-occurrences (Dubrovina et al., 2008; Granich, 2008; Ministry of Health of Ukraine, 2012). First and foremost, MMT improved 90-day treatment completion and duration of TB treatment. Absolute adherence to TB medications was also significantly higher in those receiving MMT, even after censoring for deaths and drop-outs. These findings have important implications for treating patients with at least two co-morbid conditions, specifically TB and opioid dependence, and in many cases including HIV. They support the need for integration of services previously described by Sylla et al. (2007) and recommended by international authorities (World Health Organization, 2008). Recent data from Ukraine suggest that compared to non-integrated, integrated care sites collectively result in a number of quality healthcare indicators, and also improve outcomes independently for HIV, TB or opioid dependence (Bachireddy et al., 2013).
Though a number of studies confirm that PWIDs are more likely to have latent and active TB (Deiss et al., 2009; Getahun et al., 2012), one study from New York showed that PWIDs and those who were homeless were independently associated with TB treatment non-adherence and non-adherent patients not only took longer to treat, but were more likely to develop MDR-TB (Pablos-Mendez et al., 1997). Thus, the findings from this study suggest that in the absence of MMT, PWIDs are more likely to have poorer short-term TB treatment outcomes and longer studies are warranted to determine if such differences extend beyond the 90 day observation in this study.
Also, to our knowledge, integrating MMT and DOT for active TB has not previously been examined, unlike the case for latent TB infection. In one study of patients with latent TB infection (LTBI), providing MMT with onsite DOT was associated with a four-fold improvement of isoniazid preventive treatment (IPT) completion (Batki, Gruber, Bradley, Bradley, & Delucchi, 2002). In another New York-based study, providing IPT for LTBI and DOT in MMT programs in the U.S. has been shown to be both an effective (Gourevitch, Wasserman, Panero, & Selwyn, 1996) and cost-effective TB prevention intervention (Snyder et al., 1999) among PWIDs. Description of TB treatment for patients receiving and not receiving MMT, aside from a small sample (N = 12) in the U.S. (Gourevitch et al., 1996), has not been empirically assessed until now. MMT through its reduction in illicit opioid use and related reductions in illegal behaviours and improvements in retention in care is a crucial strategy for managing TB and other co-morbid conditions among PWIDs (Altice, Kamarulzaman, Soriano, Schechter, & Friedland, 2010). Thus, these findings provide empiric support for and further galvanize international recommendations that TB services, including treatment of active TB, should be integrated with MMT programs.
A number of baseline differences were noted between our two study groups. The MMT group was older, had used drugs longer, was prescribed fewer TB medications, had longer previous hospitalization for TB, and lower use of amphetamines in the 30 days before hospitalization. In the final multivariate model where all of these differences were controlled, however, only receipt of MMT remained associated with higher retention in TB treatment. These differences raise important policy implications for future TB treatment among PWIDs.
Though buprenorphine maintenance treatment was started earlier in Ukraine (Bruce, Dvoryak, Sylla, & Altice, 2007), MMT began in 2008, and the first MMT program integrated into a TB-facility was launched in 2009. Out of 109 inpatient TB clinics in Ukraine, only 11 provide MMT, with the majority having only a few clients each. According to Ukrainian legislation, the distribution of methadone is under very strict control, and can be organized only with the permission of the Ministry of Health. Moreover, the penalties for violations of the Law on Narcotic Drugs Turnover by prescribing physicians in Ukraine are very serious (Hurley, 2010). Even minor technical mistakes made by medical staff can have serious consequences for the medics involved. Police also create additional difficulties for MMT clients (Mimiaga et al., 2010) and threaten medical staff (United States Department of State, 2011). For these reasons, the administrators of the majority of TB facilities in Ukraine prefer not to establish MMT sites within their clinics. In addition to the strict control over medical personnel, Ukrainian regulations regarding prescription of MMT require patients to have failed supervised opioid withdrawal (i.e. detoxification) at least twice previously and to be officially registered by the Narcology Centres where employment restrictions exist and driver’s licenses can be revoked (Izenberg & Altice, 2010; Bojko, Dvoriak, & Altice, 2013). As such, Ukrainian MMT patients tend to be older and with longer-term drug use. After controlling for such variables in our study, however, neither age nor duration of drug use affected retention in TB treatment. This raises the important issue of changing current regulations to relax eligibility criteria and to remove mandatory registration practices. It also highlights the need to increase the number of sites where MMT and TB treatment are co-located and more closely aligned with international standards. Research suggests that individuals dependent on opioids for two years or longer or whose opioid use severity has resulted in drug injection fare better when prescribed MMT (Amato et al., 2005).
Though not a primary outcome, the 90-day mortality of 8.2% observed in this study is exceedingly high–amortized 32.8% annual mortality since mortality occurred at all observed time points. This finding is disturbingly in line with the overall high death rate recorded in the cohorts of patients with TB in Ukraine. In 2009, up to 13% of new smear-positive and 14% of previously treated TB patients died during the course of treatment (the duration of treatment may vary, but the standard duration is six to eight months). In comparison, death rates among new and previously treated TB cases in Romania were 4% and 10%, in Poland − 5% and 5%, and in Georgia − 3% and 5%, respectively (World Health Organization, 2011). The high mortality overall may have been related to a number of factors, including co-morbid HIV infection and the high prevalence of MDR-TB (Table 1). Why mortality was significantly higher in the MMT group, however, may have been related to patients being more ill, as evidenced by a longer previous hospitalization, having been prescribed fewer or insufficient TB medications in settings with probable MDR-TB and the lack of provision of ART in the setting of HIV/TB co-infection (Table 3). Larger sample sizes and more detailed exploration of causes of death are warranted to provide further insight.
This study is the first to demonstrate that provision of MMT for patients with pulmonary TB results in improved short-term TB treatment outcomes. A number of important limitations should be noted, including a non-random sample, recruitment from among hospitalized patients, relatively small sample size and baseline differences in patients and sites. While it would have been optimal to randomize patients to MMT, this was not feasible due to ethical concerns about the need to provide MMT to all eligible patients as treatment for opioid dependence. Instead, sites were selected based on the availability of integrated care or non-availability of MMT. Despite the many differences noted between study participants in both groups, we were able to control for baseline differences in our analyses. Though the sample only included 110 study participants, to our knowledge, it is the largest observational sample examined to date of patients with pulmonary TB and opioid dependence and provides the first empirical evidence of prescribing MMT concomitant with TB treatment. Globally, most patients with pulmonary TB do not remain hospitalized for prolonged periods and this reduces the generalizability of the study findings. The TB treatment approach described here, however, is common throughout countries of the FSU in Eastern Europe and Central Asia, especially where there are emerging serious MDR-TB issues among PWIDs.
We were unable to disentangle the impact of MMT retention or MMT dose on TB treatment outcomes for a number of reasons. First, MMT adherence among those prescribed was essentially 100%. Second, while it would be seductive to stratify by MMT dose, (dose ranged between 12 and 225 mg), it is well-known that rifampicin markedly reduces methadone levels (Altice et al., 2010; Niemi, Backman, Fromm, Neuvonen, & Kivisto, 2003) and not all patients were on rifampicin due to high levels of MDR-TB. Thus, future studies should disentangle the impact of quality of prescription of MMT on TB treatment outcomes either through enrolment of larger sample sizes and/or measuring the impact of methadone dose on TB treatment outcomes.
Despite the social stabilization intended through hospitalization of patients who would otherwise be deemed at high risk for discontinuing therapy, this study demonstrated significantly improved TB-related outcomes by providing MMT in addition to addressing other destabilizing factors like homelessness. As such, our findings represent the most conservative possible outcome given the added stability found in a supervised setting. Future studies should examine patients who transition from hospitals to community to determine if MMT continues to improve outpatient TB treatment outcomes. Also important is examination of the reduction within hospital of TB transmission, to compare with TB treatment outcomes for those whose TB is managed simultaneously through provision of MMT in community-based settings. Similar studies of HIV-infected opioid dependent prisoners who are transitioning to community affirm the benefit of buprenorphine treatment in improving HIV treatment outcomes (Springer, Qiu, Saber-Tehrani, & Altice, 2012). Also, integration of buprenorphine into HIV treatment settings improves a number of HIV treatment outcomes, including receipt of ART, improvements in CD4 and levels of viral suppression (Altice et al., 2011). Together, these findings build on previous work where simultaneous management of both conditions optimizes the effectiveness of treatment. Importantly, we cannot comment whether organizational factors associated with integrating MMT into TB treatment settings may explain some of the outcomes. Organizational issues for MMT, HIV and TB integration have been previously described (Sylla et al., 2007) and recent data suggest improved quality health indicators for all three conditions when MMT is provided in integrated treatment settings in Ukraine (Bachireddy et al., 2013).
Another topic for future research is the overall assessment of the quality and appropriateness of TB treatment in Ukraine, with a focus on PWIDs and especially those who are co-infected with HIV. Based on the observations from this study, we hypothesize that the quality of TB treatment might undermine health outcomes of such patients. Notwithstanding these limitations, this study provides empiric support for providing MMT (or other evidence-based treatment for opioid dependence) for TB patients who also have opioid dependence. For MMT to provide benefit, however, it must be expanded more broadly to patients in TB treatment settings and to those who do not currently meet extremely stringent criteria for MMT in Ukraine.
Conclusions
The study demonstrates that MMT integrated with inpatient TB treatment significantly improves retention in TB treatment and adherence to TB medications among PWIDs. After controlling for potential confounders, the receipt of MMT remained the only predictor of improved 90-day TB treatment completion. These results are generally in line with the findings about the role of MMT in improving adherence to HIV treatment and treatment of LTBI among PWIDs elsewhere. The results of this study highlight a need for policy changes to increase the number of MMT sites available within TB treatment facilities and to make MMT a low-threshold treatment option for opioid dependence for TB patients in Ukraine.
Acknowledgments
The authors express their gratitude to the study participants and the staff of the Mykolayiv, Odessa, Kherson, Donetsk, Dnipropetrovsk and Kyiv Tuberculosis clinics for taking part in the implementation of the study.
Funding
This study was supported by the grant from the Open Society Foundation, New York City (#20033135) and through career development (K24 DA017072) and research (R01 DA029910 and R01 DA033679) from the National Institutes for Drug Abuse for FLA.
Footnotes
Author contributions
S.D. and O.M. conceived and designed the experiments; S.D. performed the experiments; O.M. and F.L.A. analysed and interpreted the data; O.M., F.L.A., and S.D. wrote the paper.
Conflict of interest statement
The authors report no conflict of interests.
References
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: Results from a multisite study. Journal of Acquired Immune Deficiency Syndromes. 2011;56(Suppl. 1):S22–S32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment. 2005;28(4):321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug and Alcohol Dependence. 2013 doi: 10.1016/j.drugalcdep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug and Alcohol Dependence. 2002;66:283–293. doi: 10.1016/s0376-8716(01)00208-3. [DOI] [PubMed] [Google Scholar]
- Bojko MJ, Dvoriak S, Altice FL. At the crossroads: HIV prevention and treatment for people who inject drugs in Ukraine. Addiction. 2013;108(10):1697–1699. doi: 10.1111/add.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine – programme description and policy implications. International Journal of Drug Policy. 2007;18(4):326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss RG, Rodwell TC, Garfein RS. Tuberculosis and illicit drug use: Review and update. Clinical Infectious Diseases. 2009;48(1):72–82. doi: 10.1086/594126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovina I, Miskinis K, Lyepshina S, Yann Y, Hoffmann H, Zaleskis R, et al. Drug-resistant tuberculosis and HIV in Ukraine: A threatening convergence of two epidemics? International Journal of Tuberculosis and Lung Disease. 2008;12(7):756–762. [PubMed] [Google Scholar]
- Getahun H, Gunneberg C, Sculier D, Verster A, Raviglione M. Tuberculosis and HIV in people who inject drugs: Evidence for action for tuberculosis, HIV, prison and harm reduction services. Current Opinion in HIV and AIDS. 2012;7(4):345–353. doi: 10.1097/COH.0b013e328354bd44. [DOI] [PubMed] [Google Scholar]
- Glaziou P, Floyd K, Raviglione M. Global burden and epidemiology of tuberculosis. Clinics in Chest Medicine. 2009;30(4):621–636. doi: 10.1016/j.ccm.2009.08.017. vii. [DOI] [PubMed] [Google Scholar]
- Gourevitch MN, Wasserman W, Panero MS, Selwyn PA. Successful adherence to observed prophylaxis and treatment of tuberculosis among drug users in a methadone program. Journal of Addictive Diseases. 1996;15(1):93–104. doi: 10.1300/J069v15n01_07. [DOI] [PubMed] [Google Scholar]
- Granich R. HIV and MDR-TB in Ukraine: News from a hazardous MDR-TB and HIV site. International Journal of Tuberculosis and Lung Disease. 2008;12(7):701. [PubMed] [Google Scholar]
- Hurley R. How Ukraine is tackling Europe’s worst HIV epidemic. British Medical Journal. 2010;341:c3538. doi: 10.1136/bmj.c3538. [DOI] [PubMed] [Google Scholar]
- Izenberg JM, Altice FL. Next steps for Ukraine abolition of HIV registries, implementation of routine human immunodeficiency virus testing and expansion of services. Addiction. 2010;105(3):569–570. doi: 10.1111/j.1360-0443.2009.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janca A, Ustun TB, Early TS, Sartorius N. The ICD-10 symptom checklist: A companion to the ICD-10 classification of mental and behavioural disorders. Social Psychiatry and Psychiatric Epidemiology. 1993;28(5):239–242. doi: 10.1007/BF00788743. [DOI] [PubMed] [Google Scholar]
- Kruglov YV, Kobyshcha YV, Salyuk T, Varetska O, Shakarishvili A, Saldanha VP. The most severe HIV epidemic in Europe: Ukraine’s national HIV prevalence estimates for 2007. Sexually Transmitted Infections. 2008;84(Suppl 1):i37–i41. doi: 10.1136/sti.2008.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk A, Bannister W, Podlekareva DN, Chentsova NP, Rakhmanova AG, Hor-ban A, et al. Tuberculosis among HIV-positive patients across Europe: Changes over time and risk factors. AIDS. 2011;25(12):1505–1513. doi: 10.1097/QAD.0b013e328348fafd. [DOI] [PubMed] [Google Scholar]
- Mahmoudi A, Iseman MD. Pitfalls in the care of patients with tuberculosis. Common errors and their association with the acquisition of drug resistance. Journal of the American Medical Association. 1993;270(1):65–68. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Safren SA, Dvoryak S, Reisner SL, Needle R, Woody G. “We fear the police, and the police fear us”: Structural and individual barriers and facilitators to HIV medication adherence among injection drug users in Kiev, Ukraine. AIDS Care. 2010;22(11):1305–1313. doi: 10.1080/09540121003758515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health of Ukraine. Ukraine: Harmonized AIDS response progress report. Kyiv: ICF “International HIV/AIDS Alliance in Ukraine”; 2012. reporting period: January 2010–December 2011. [Google Scholar]
- Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clinical Pharmacokinetics. 2003;42(9):819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Nonadherence in tuberculosis treatment: Predictors and consequences in New York City. American Journal of Medicine. 1997;102(2):164–170. doi: 10.1016/s0002-9343(96)00402-0. [DOI] [PubMed] [Google Scholar]
- Snyder DC, Paz EA, Mohle-Boetani JC, Fallstad R, Black RL, Chin DP. Tuberculosis prevention in methadone maintenance clinics. Effectiveness and cost-effectiveness. American Journal of Respiratory and Critical Care Medicine. 1999;160(1):178–185. doi: 10.1164/ajrccm.160.1.9810082. [DOI] [PubMed] [Google Scholar]
- Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One. 2012;7(5):e38335. doi: 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story A, Murad S, Roberts W, Verheyen M, Hayward AC, London Tuberculosis Nurses Network. Tuberculosis in London: The importance of homelessness, problem drug use and prison. Thorax. 2007;62(8):667–671. doi: 10.1136/thx.2006.065409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. International Journal of Drug Policy. 2007;18(4):306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of State. Country reports on human rights practices for 2011. Ukraine: Bureau of Democracy, Human Rights and Labor; 2011. [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global tuberculosis control 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- World Health Organization, UNODC, & UNAIDS. Policy guidelines for collaborative TB and HIV services for injecting and other drugusers: An integrated approach. Geneva: World Health Organization/UNODC/UNAIDS; 2008. [PubMed] [Google Scholar]


