ABSTRACT
Infant humans and rhesus macaques infected with the human or simian immunodeficiency virus (HIV or SIV), respectively, express higher viral loads and progress more rapidly to AIDS than infected adults. Activated memory CD4+ T cells in intestinal tissues are major primary target cells for SIV/HIV infection, and massive depletion of these cells is considered a major cause of immunodeficiency. Monocytes and macrophages are important cells of innate immunity and also are targets of HIV/SIV infection. We reported previously that a high peripheral blood monocyte turnover rate was predictive for the onset of disease progression to AIDS in SIV-infected adult macaques. The purpose of this study was to determine if earlier or higher infection of monocytes/macrophages contributes to the more rapid progression to AIDS in infants. We observed that uninfected infant rhesus macaques exhibited higher physiologic baseline monocyte turnover than adults. Early after SIV infection, the monocyte turnover further increased, and it remained high during progression to AIDS. A high percentage of terminal deoxynucleotidyltransferase dUTP nick end label (TUNEL)-positive macrophages in the lymph nodes (LNs) and intestine corresponded with an increasing number of macrophages derived from circulating monocytes (bromodeoxyuridine positive [BrdU+] CD163+), suggesting that the increased blood monocyte turnover was required to rapidly replenish destroyed tissue macrophages. Immunofluorescence analysis further demonstrated that macrophages were a significant portion of the virus-producing cells found in LNs, intestinal tissues, and lungs. The higher baseline monocyte turnover in infant macaques and subsequent macrophage damage by SIV infection may help explain the basis of more rapid disease progression to AIDS in infants.
IMPORTANCE HIV infection progresses much more rapidly in pediatric cases than in adults; however, the mechanism for this difference is unclear. Using the rhesus macaque model, this work was performed to address why infants infected with SIV progress more quickly to AIDS than do adults. Earlier we reported that in adult rhesus macaques, increasing monocyte turnover reflected tissue macrophage damage by SIV and was predictive of terminal disease progression to AIDS. Here we report that uninfected infant rhesus macaques exhibited a higher physiological baseline monocyte turnover rate than adults. Furthermore, once infected with SIV, infants displayed further increased monocyte turnover that may have facilitated the accelerated progression to AIDS. These results support a role for monocytes and macrophages in the pathogenesis of SIV/HIV and begin to explain why infants are more prone to rapid disease progression.
KEYWORDS: AIDS, macrophages, pediatric infectious disease, simian immunodeficiency virus
INTRODUCTION
The use of antiretroviral therapies (ART) has dramatically reduced mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) infection in developed countries (1–3). However, there are still many obstacles to preventing MTCT in developing countries where ART is unavailable and few alternatives to breast-feeding exist. Neonatal HIV infections are often associated with a more rapid disease progression and a higher mortality rate than with adult infections. Two distinct patterns of disease progression are observed in untreated, HIV-infected children. In the first pattern, approximately 50% of children develop serious disease and die by 2 years of age (4, 5). In the remaining group, infected children express significantly higher levels of virus replication than adults, develop a slower onset of disease, and may survive until adolescence (6). A better understanding of the mechanisms underlying the pathogenesis and risk factors associated with HIV infection that are unique to neonates and young children is essential for the development of effective intervention strategies.
HIV and simian immunodeficiency virus (SIV) target activated memory CCR5+ CD4+ T cells that become rapidly depleted during the acute phase, especially in mucosal tissues such as the intestine (7–9). After an initial immune response that reduces viral load and partially restores CD4+ T cell levels in adults, virus persistence and the slow continuous decline of CD4+ T cells eventually contribute to terminal disease progression to AIDS, with a median survival of 11 years without treatment (9, 10). In contrast to the case with adults, CCR5+ CD4+ T cells are dramatically absent in the blood and lymph nodes (LNs) of infants (11), and HIV/SIV infections in human and rhesus macaque infants progress more rapidly than in adults, with onset of AIDS within 2 years and onset of simian AIDS by 6 months of age, respectively (12, 13). Rhesus infants did exhibit an increased number of activated memory CCR5+ CD4+ T cells in the gut, and these cells serve as major targets of SIV infection, resulting in their subsequent depletion (11, 13, 14). In addition, these intestinal CD4+ T cells exhibited a markedly high proliferating capability (13). Despite the presence of this CCR5+ CD4+ T cell subset in the gut, the majority of CD4+ T cells in neonates exhibit a naive phenotype and are skewed functionally toward immune tolerance (14–18). In light of these phenotypic and functional differences, along with the higher CD4+ T cell levels in children than in adults, the age-related declines of CD4+ T cells in infants (19–21), an overall lower number of activated memory target cells in neonates than in adults, and no strong evidence indicating higher systemic CD4+ T cell depletion in infants (22–24), it remains unclear what is driving the faster and more severe disease progression in pediatric cases. This highlights the importance of further defining T cell immune responses in HIV progression as well as the response of other immune cells that are targeted by infection such as macrophages.
Relatively less is known about the contribution of monocytes and macrophages to AIDS pathogenesis. Macrophages can express the receptors necessary for viral entry of HIV, including CD4, CCR5, and CXCR4, and macrophages support infection in vivo to various degrees based on tissue sites (25, 26). Also, we reported previously that increased monocyte turnover better predicts onset of terminal disease progression in adults than declining levels of CD4+ T cells alone (27, 28). Furthermore, we observed increased monocyte trafficking from bone marrow to blood and their subsequent differentiation into macrophages in peripheral tissues, including LNs, central nervous system, and lung, during SIV infection in adult rhesus macaques (29–31). This increase in monocyte turnover, recruitment, and differentiation correlated with the rate of progression to AIDS, severity of lung tissue damage, and SIV encephalitis (29–31). Monocytes and tissue macrophages participate in innate immune responses via recognition and phagocytosis of invading pathogens and secretion of inflammatory cytokines and chemokines. Macrophages also serve as targets of HIV/SIV infection and contribute to pathogenesis as represented by giant cell pneumonia and AIDS encephalitis during late-stage infection (30, 32, 33). Furthermore, massive macrophage infection is observed in cases of rapid AIDS progression with sustained high viral loads during SIV/SHIV infection of adult macaques, as well as in SIV-infected rhesus macaques depleted of CD4+ T cells (29, 30). Taken together, the findings led us to hypothesize that the rapid AIDS progression in HIV/SIV-infected infants is associated with macrophage infection and destruction followed by increasing monocyte turnover to recruit and replace the damaged tissue macrophages. The purpose of this study was to characterize the frequencies and phenotypes of monocytes and macrophages during AIDS disease progression in newborn and infant rhesus macaques infected with SIVmac251. The results indicated that in addition to CD4+ T cells, infection of monocytes and macrophages was critical for rapid progression to AIDS in pediatric cases.
RESULTS
High baseline monocyte turnover in uninfected infants compared to adult rhesus macaques.
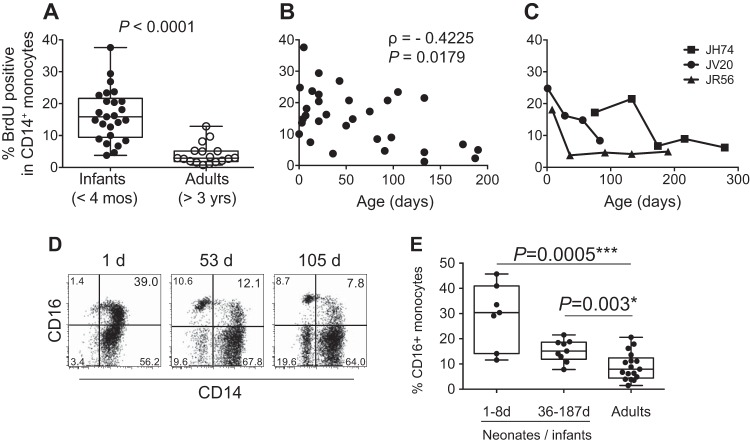
To compare baseline blood monocyte turnover, 5′-bromo-2′-deoxyruridine (BrdU) or 5-ethynyl-2′deoxyuridine (EdU) was administered by intravenous (i.v.) injection to young rhesus macaques aged 2 to 189 days (Table 1). Blood samples were collected 24 h later (or as otherwise noted) to detect BrdU incorporation by dividing cells to measure monocyte turnover using flow cytometry as previously described (28). As shown in Fig. 1A, the median monocyte turnover in the uninfected neonate and infant macaques was 15.9% (lower and upper quartile range, 9.46% to 21.65%), compared to 2.9% (lower and upper quartile range, 1.84% to 5.15%) in the uninfected adults, which was statistically significantly higher (Mann-Whitney U test, P < 0.0001, two-tailed t test). Furthermore, 19 out of 25 (76%) of the uninfected infants exhibited greater than 10% BrdU+ monocytes (CD3− CD20− CD8− HLA-DR+ CD14+), compared to only 1 of 15 (6.7%) uninfected adults. An inverse correlation also was observed between decreasing monocyte turnover and increasing age until reaching 100 to 120 days of age (r = −0.4225; P = 0.012), indicating that a normal adult physiological monocyte turnover appeared to develop at about 3 months of age (Fig. 1B). For further corroboration, monocyte turnover was followed longitudinally in three infant macaques. Over the span of a year, BrdU was administered every 4 to 6 weeks, followed by flow cytometric monitoring of monocyte turnover 24 h after each BrdU injection based on monocyte BrdU incorporation kinetics established in earlier studies (28). Monocyte turnover rates after birth ranged from 18.1 to 24.8% and decreased to 5 to 8% in all animals within 6 months of age (Fig. 1C).
TABLE 1.
Summary of study pediatric rhesus macaquesa
| Animal ID | BrdU data for UI animals | Age (days) when BrdU data were obtained for UI animals | Age (days) at SIV infection | No. of wks PI when BrdU or EdU data were obtained |
|---|---|---|---|---|
| GN99 | ✓ | 3 | ||
| JR85 | ✓ | 4 | ||
| JT32 | ✓ | 8 | ||
| GB15 | ✓ | 12 | ||
| GA98 | ✓ | 14 | ||
| EC73 | ✓ | 21 | ||
| DP44 | ✓ | 21 | ||
| JV94 | ✓ | 21 | ||
| JV96 | ✓ | 21 | ||
| JG22 | ✓ | 43 | ||
| GA24 | ✓ | 50 | ||
| IA09 | ✓ | 132 | ||
| IG61 | ✓ | 134 | ||
| IG58 | ✓ | 137 | ||
| GB18 | ✓ | 186 | ||
| HT46 | ✓ | 187 | ||
| JR56 | ✓ | 8, 36, 91, 133, 190 | ||
| JV20 | ✓ | 1, 29, 57, 83 | ||
| JH74 | ✓ | 75, 133, 174, 378 | ||
| FT82 | 1 | 0 | ||
| FP86 | 0 | 1 | ||
| HC17 | 1 | 1 | ||
| GG94 | 1 | 1 | ||
| GL48 | 1 | 2 | ||
| GN84 | 1 | 2 | ||
| FP81 | 0 | 2 | ||
| GP20 | 1 | 3 | ||
| DP53 | 0 | 3 | ||
| DP62 | 0 | 3 | ||
| GL06 | 1 | 10 | ||
| HP55 | 1 | 11 | ||
| GH11 | 1 | 15 | ||
| JL94 | 0 | 0, 4, 6, 10 | ||
| JR01 | 0 | 0, 4, 5 | ||
| KI13 | ✓ | 4 | 5 | |
| JF87 | ✓ | 53 | 78 | 2, 8, 14, 22 |
| JG94 | ✓ | 94 | 136 | 2, 8, 14, 23, 31, 37, 43, 49, 55, 63, 71, 75 |
| JD31 | ✓ | 98 | 123 | 2, 8, 14, 22, 30, 47 |
| JA96 | ✓ | 105 | 130 | 2, 8, 11, 30 |
ID, identifier; UI, uninfected; PI, postinfection.
FIG 1.
High monocyte turnover in uninfected infant rhesus macaques. BrdU incorporation was analyzed at 24 h by multicolor flow cytometry to determine the percentage of BrdU+ monocytes (HLA-DR+ CD3− CD8− CD20− CD14+). Monocyte turnover rates were compared between infant rhesus macaques and adult rhesus macaques (A), examined in 20 uninfected macaques ranging from 3 to 190 days of age (B), and monitored longitudinally in three rhesus macaques every 4 to 6 weeks (C). The frequency of CD16+ CD14+ monocytes was analyzed (D and E). (D) Representative flow cytometry data in infant rhesus macaques. (E) Comparison of the frequency of CD16+ CD14+ monocytes among neonates, older infants, and adults. Measures of statistical significance were assessed by two-tailed Mann-Whitney U test (A) and Spearman correlation analysis (B) and Kruskal-Wallis test corrected for multiple comparisons using Dunn's posttest (E). *, P < 0.05; ***, P < 0.001.
During homeostasis, the majority of monocytes from humans and rhesus macaques stain CD14+ CD16− (34), but CD16 expression on monocytes increases under inflammatory conditions (35–37). We thus investigated the frequency of CD16-expressing monocytes as an indirect indicator of monocyte activation in infant macaques. CD16 expression was higher in the animals aged 1 to 8 days old (median = 30.39%; lower and upper quartile range, 14.11 to 40.97%) than in older infants (median = 15.14%; lower and upper quartile range, 12.32 to 18.66%) and adults (median = 7.92%; lower and upper quartile range, 4.43 to 12.4%) (Fig. 1D and E).
SIV infection in infant macaques led to further increases in monocyte turnover and rapid progression to AIDS.
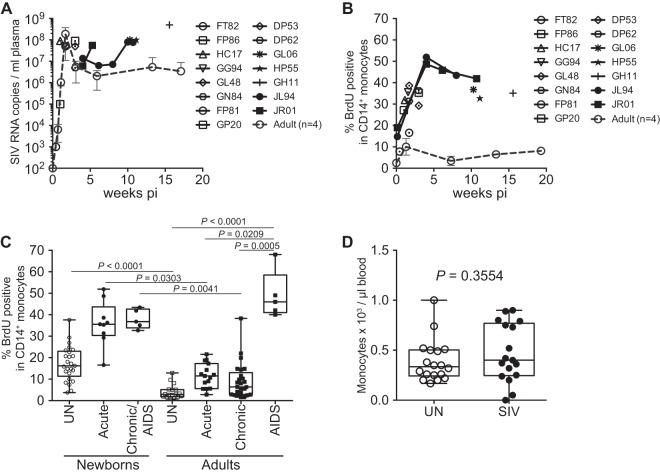
Since increased monocyte turnover correlated with AIDS disease progression in SIV-infected adult rhesus macaques (28), we examined whether higher initial baseline monocyte turnover rates would be reflected in the rapid disease progression in infants. Newborn rhesus macaques were inoculated i.v. with a pathogenic SIV strain (SIVmac251) within 24 h of birth and euthanized at specified time points regardless of disease progression status (Fig. 2A and B; Table 1). Two of those animals, JL94 and JR01, were monitored to confirm an overview of disease progression depicted by single time point from each infected neonate until exhibiting AIDS-related clinical signs (e.g., weight loss and diarrhea) and euthanized on days 75 and 56 postinfection, respectively (Fig. 2A and B). Consistent with previously reported studies (13, 14) peak plasma viral loads in the newborn macaques were greater than 107 SIV RNA copies/ml plasma and levels of virus remained high throughout the course of infection (Fig. 2A).
FIG 2.
SIV infection in newborn macaques induced rapid increases in monocyte turnover and disease progression to AIDS. (A) Plasma viral loads (PVL) in 15 infant macaques infected within 24 h of birth as well as the mean PVL of four infected adult macaques (dashed line) were plotted. (B) BrdU incorporation was evaluated for monocyte turnover at 24 h. Blood monocyte turnover rates were measured every 4 to 6 weeks during the course of infection in neonates JL94 (solid circles) and JR01 (solid squares) until progression to AIDS, and at necropsy in 13 other infected macaques (open symbols). Mean monocyte turnover rates (i.e., percent BrdU+ CD14+ monocytes) of the four infected adults are shown for comparison (dashed line). (C) Statistical significance of comparisons between monocyte turnover rates of newborn and adult rhesus macaques before infections (UN, uninfected) and during acute, chronic, and AIDS stages of SIV-infected macaques were determined by Kruskal-Wallis test corrected for multiple comparisons using Dunn's posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) Absolute counts of monocytes in uninfected and SIV-infected infant macaques were compared by Mann-Whitney U test and were not significantly different.
In vivo BrdU pulse studies were performed to compare monocyte turnover in adult and newborn rhesus macaques at time points before infection, during acute, chronic, and AIDS stages of SIV infection, and at euthanasia. At peak viremia during the acute phase, or approximately 2 weeks after SIV infection, the monocyte turnover rates were significantly higher in newborns, with a median of 35.6% (Fig. 2B and C), compared to a median of 11.5% in four infected adults (P = 0.0303). The high monocyte turnover rate observed in neonates during acute infection was equivalent to levels reached only during the terminal stages of AIDS in the SIV-infected adult macaques, which exhibited a 46% median monocyte turnover rate (Fig. 2C) (28).
Newborns differed from adult macaques in the kinetics of monocyte turnover during the overall course of infection. Prior to infection, the baseline monocyte turnover was already significantly higher in the neonates (median = 16.2%) than in the adults (median = 2.9% [Fig. 2B and C]). The monocyte turnover increased further after SIV infection in the infected neonates during the acute peak viremia stage and during progression to AIDS (median = 36.8%). In contrast, the monocyte turnover in infected adults returned to near baseline levels after peak viremia acute stage, slightly increased during the chronic stage (median = 6.43%), and then increased significantly only during AIDS (Fig. 2C). In general, onset of AIDS-related clinical signs in the SIV-infected adult macaques occurred when the monocyte turnover rate reached ∼20%. In contrast, the turnover rate in the neonates originated at ∼20% and further increased after infection. Interestingly, the absolute numbers of blood monocytes were not significantly different before or after SIV infection in the newborn macaques, despite increased output of monocytes from bone marrow (Fig. 2D). Furthermore, the absolute number of monocytes in the neonates and infants were similar to numbers found in adult rhesus macaques in previous work (28).
Increased accumulation and apoptosis of CD163+ macrophages in the LNs of SIV-infected newborn macaques.
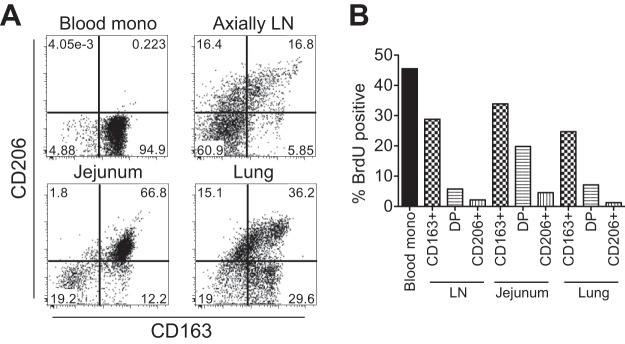
We then investigated if circulating monocytes undergoing physiologically higher turnover in uninfected neonates were massively migrating into peripheral tissues. Tissues from axillary LNs, intestines (jejunum), and lungs were collected from an uninfected neonate at necropsy that was performed 48 h after the final BrdU i.v. injection to enable detection of migrating and differentiating monocytes. Blood monocytes and tissue macrophages were identified for expression of CD163 (scavenger receptor) and CD206 (mannose receptor) (Fig. 3). Blood monocytes expressed the phenotype CD163+ CD206−, and over 40% stained for BrdU incorporation 48 h after BrdU administration. Tissue macrophages were contained in the lineage-negative HLA-DR+ fraction, and at least three distinct populations were identified by CD163 and CD206 expression: CD163+ CD206−, CD163+ CD206+, and CD163− CD206+. As described on the basis of our previous work using adult rhesus macaques (35), the majority of the BrdU+ cells were in the CD163+ CD206− population, suggesting that these macrophages most recently differentiated from immigrating monocytes. Fewer BrdU+ cells stained CD163+ CD206+ and appeared to be mature and/or differentiated macrophages. Finally, the CD163− CD206+ cells expressed CD11bdim/low, in contrast to high CD11b expression in the other two populations (CD163+ CD206− and CD163+ CD206+), exhibited negligible staining with BrdU, and thus were considered to be tissue resident macrophages or dendritic cells, as reported previously (38–42).
FIG 3.
Phenotype and turnover of tissue macrophages in an uninfected rhesus macaque. A rhesus macaque was injected with BrdU at 5 days of age and necropsied at 7 days of age. (A) Isolated cells from tissues were analyzed by flow cytometry. The HLA-DR+ fraction was analyzed for CD163 and CD206 expression. (B) The percentage of each monocyte/macrophage subset that incorporated BrdU from each tissue was graphed in comparison to blood monocyte turnover.
We next investigated the kinetics of intestinal macrophage population shifts in SIV-infected compared to uninfected infants by flow cytometry. Intestinal tissues are considered a primary site of HIV/SIV infection and exhibit dramatic pathological changes throughout the course of infection (7, 8). The majority of macrophages were identified as the CD163+ CD206+ cells in the CD11bhigh HLA-DR+ cell fraction (Fig. 4A) and were localized in the lamina propria of the intestinal tissues (Fig. 4B). A small proportion of CD163+ CD206− macrophages was detected in the intestinal tissues of naive infants, as well (Fig. 3A and 4C). Early after SIV infection (12 days postinfection) in the newborn macaques, there was a dramatic decline in CD163+ CD206+ macrophages in both the jejunum and colon (Fig. 4C), similar to the depletion of this macrophage cell subset that occurs in infected adults at the terminal stage of AIDS and briefly during the acute stage. In infants before and during SIV infection, there was a direct correlation between the percentages of CD163+ CD206− macrophages and blood monocyte turnover rates, suggesting that these macrophages recently differentiated from the immigrating blood monocytes.
FIG 4.
Loss of CD163+ CD206+ macrophages and accumulation of EdU+ CD163+ macrophages in intestinal tissues after SIV infection in infant macaques. (A) Intestinal macrophages were identified by multicolor flow cytometry. CD3+ and CD20+ cells were excluded and macrophages were selected based on staining HLA-DR+ CD11b+. (B) Confocal microscopy demonstrated that the majority of macrophages in the lamina propria stained CD163+ CD206+. (C) Cells were analyzed for CD163 and CD206 expression, and the double-positive cells were identified as the intestinal macrophages. Intestinal macrophage phenotypes were compared in uninfected and SIV-infected infant macaques. A loss of CD163+ CD206+ and accumulation of CD163+ CD206− macrophages were observed to occur in the jejunum and colon after SIV infection. This accompanied an increase in the percentage of CD163+ CD206− macrophages in the jejunum and colon that incorporated EdU.
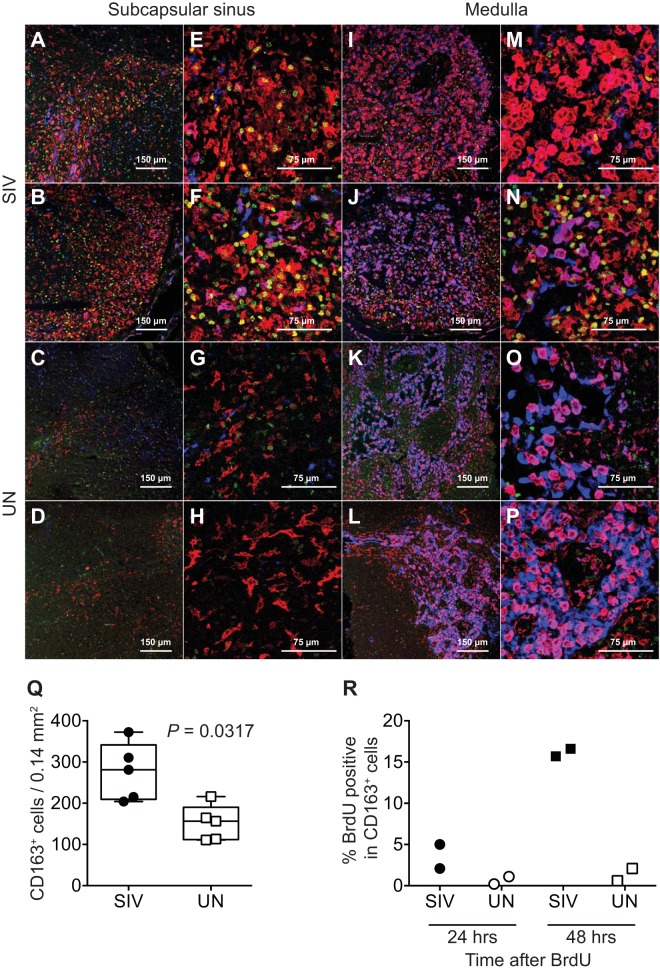
In axillary LNs, CD163+ CD206− macrophages were localized in the subcapsular sinus, while the CD163+ CD206+ macrophages and CD163− CD206+ macrophages were localized in the medulla (Fig. 5A to P). The number of CD163+ CD206− macrophages in the subcapsular sinus regions of the axillary LNs of SIV-infected infants with massive monocyte turnover was significantly higher (median = 281/0.14-mm2 area) than in the uninfected infants (median = 156.4/0.14-mm2 area) (Mann-Whitney U test, P = 0.0317, two tailed) (Fig. 5A to H and Q). In the medulla of the axillary LNs of SIV-infected infants, no significant change was observed in the number of CD163+ CD206+ macrophages compared to that of uninfected infants (Fig. 5M to P).
FIG 5.
CD163+ macrophages accumulated in LNs of SIV-infected infants. Tissue sections of axillary LNs were examined by immunohistochemistry using antibodies to detect BrdU (green), CD163 (red), and CD206 (blue). Representative confocal images of subcapsular and medullar regions of LNs are shown for two SIV-infected infants (A, E, I, and M for infant JL94 and B, F, J, and N for infant JR01) and two uninfected naive infants (C, G, K, and O for infant JR56 and D, H, L, and P for infant JV20). Images in panels A to D and I to L are at a magnification of ×20, and those in panels E to H and M to P are at a magnification of ×40. (Q) Significant differences were detected between the numbers of CD163+ cells per field in LNs of SIV-infected compared to uninfected infants by two-tailed Mann-Whitney U test. (R) Dividing cells in LNs were detected 24 and 48 h after BrdU injections. Numbers of BrdU+ CD163+ macrophages were reported as a percentage of total CD163+ macrophages.
More BrdU (green)-labeled cells also were observed in the LNs of SIV-infected infants, and the majority of BrdU+ macrophages were CD163+ CD206− (Fig. 5). Since BrdU incorporation is a marker of dividing cells, it is likely that BrdU+ CD163+ CD206− cells proliferated locally as a consequence of tissue activation or inflammation (43). Therefore, we injected two of the SIV-infected infants with BrdU 24 h prior to necropsy to identify recently divided cells. Only 2.1% and 5.0% of the CD163+ CD206− macrophages incorporated BrdU, even though the blood monocyte turnover rates were very high, at 39.3% and 42.2%, respectively (Fig. 5R). In two SIV-infected infants administered BrdU 48 h prior to necropsy, BrdU incorporation by CD163+ CD206− macrophages was dramatically higher, at 16.6% and 15.7% (Fig. 5R). These results suggest that the accumulation or increased number of CD163+ CD206− macrophages in the axillary lymph nodes resulted from recently immigrating monocytes accompanied by increased monocyte turnover in the blood of SIV-infected infants.
To investigate the fate of accumulated macrophages and the mechanism of high monocyte turnover, the rate of tissue macrophage cell death was analyzed by staining for terminal deoxynucleotidyltransferase dUTP nick end labeling (TUNEL) in the LNs obtained from SIV-infected and uninfected infants (Fig. 6A to F). The percentage of TUNEL+ CD163+ cells observed in the SIV-infected (median = 10.5%) compared to uninfected infants (median = 0%) was significantly higher (Mann-Whitney U test, P = 0.0079, two tailed), and there also was a direct correlation between the percentage of TUNEL+ CD163+ cells and numbers of CD163+ cells in LNs, providing a direct link between increased death rate of tissue macrophages and higher blood monocyte turnover (Fig. 6G and H).
FIG 6.
Increased apoptosis of CD163+ CD206− early macrophages was observed in LNs of SIV-infected infant macaques. Axillary or inguinal LNs were stained for TUNEL (green), CD163 (red), and CD206 (blue). Representative images are shown for two SIV-infected infants (JL94 in panels A and C and JR01 in panels B and D) and two uninfected infants (JV20 in panel E and EC72 in panel F). (G) Mann-Whitney U test (two tailed) was used to compare TUNEL+ CD163+ cells in LNs of uninfected (UN) and SIV-infected infants. (H) Spearman analysis demonstrated a statistically significant direct correlation between the total numbers of CD163+ macrophages and percentage of TUNEL+ CD163+ macrophages in LNs of infected infant macaques.
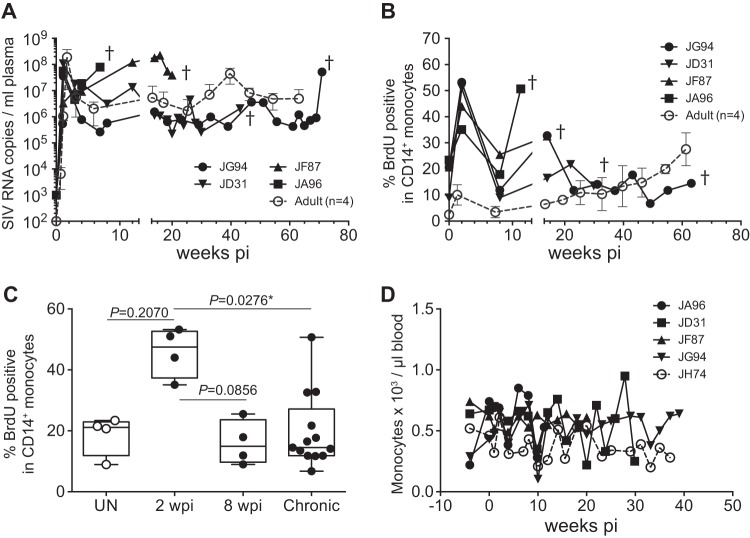
Infant macaques infected at 3 to 4 months of age showed an intermediate disease progression phenotype compared to those of newborns and adults.
We next compared findings from neonatal infected macaques expressing persistent high plasma viral loads and high monocyte turnover rates to results from infant macaques infected at 3 to 4 months of age, when physiological monocyte turnover rate was declining and transitioning toward adult physiological monocyte turnover rates (Fig. 1C). Prior to infection, four infants at 3 to 4 months of age had monocyte turnover rates ranging from 8.92% to 23.4% (median of 21.1%; lower and upper quartile range, 11.87% to 22.93%) (Fig. 7B and C). Of these four infants, JA96 and JF87 showed plasma viral loads similar to those of newborns and progressed to AIDS by 20 weeks after infection (Fig. 7A). In the remaining two infants (JD31 and JG94), plasma SIV loads more closely resembled those seen in adults, and their infections transitioned to the chronic phase rather than progressing directly to overt AIDS (Fig. 7A). JD31 died from a non-SIV/AIDS-related condition at 30 weeks, and JG94 was euthanized 75 weeks after infection without yet exhibiting AIDS-related clinical signs. Monocyte turnover after SIV infection in the 3- to 4-month-old infants dramatically increased, reaching a median of 47.5% (lower and upper quartile range, 37.33% to 52.65%) during the acute phase (2 weeks postinfection), which was similar to that seen in the infected newborns, but subsequently declined to a median of 14.9% (lower and upper quartile range, 9.71% to 23.6%) (Fig. 7B and C) at 8 weeks postinfection. The monocyte turnover increased again in the infants infected at 3 to 4 months of age, when they were exhibiting clinical signs associated with AIDS (Fig. 7B). Similar to the data obtained for newborns, increases in monocyte turnover were not reflected in absolute number of circulating monocytes (Fig. 7D).
FIG 7.
Longitudinal course of changes observed during progression to AIDS in infant macaques infected with SIVmac251 at 3 to 4 months of age. (A) PVL in four infant macaques infected with SIVmac251 were plotted as well as the mean PVL of four infected adult macaques (dashed line). (B) BrdU incorporation was evaluated for monocyte turnover at 24 h. Blood monocyte turnover rates were measured every 4 to 6 weeks during the course of infection until progression to AIDS or at indicated time points (dagger). Mean monocyte turnover rates (i.e., percent BrdU+ CD14+ monocytes) of the four infected adults are shown for comparison (dashed line). (C) Statistical analysis of monocyte turnover rates in infant macaques was performed by Kruskal-Wallis test corrected for multiple comparisons using Dunn's posttest. *, P < 0.05. UN, preinfection; pi, postinfection. (D) Absolute counts of monocytes.
Increased infection of macrophages occurred in SIV-infected infants expressing elevated monocyte turnover.
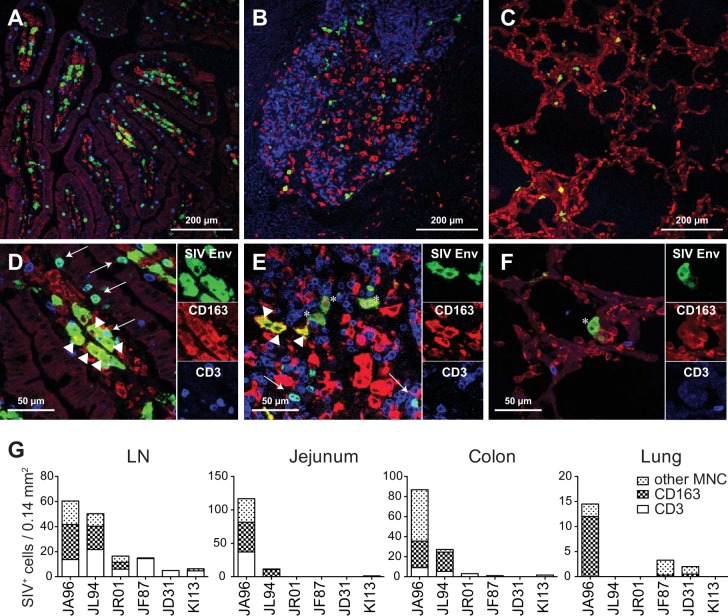
Immunohistochemistry was then applied to detect SIV replication within CD3+ T cells and CD163+ macrophage cell subsets in the LNs, intestines, and lungs of six SIV-infected newborns and infant macaques (Fig. 8). Three of these infected infant macaques, JA96, JL94, and JR01, were rapid progressors, with onset of disease occurring by 11 weeks postinfection, while the remaining animals, JF87, JD31, and KI13, survived longer than 6 months and/or died due to non-AIDS-related causes. SIV-infected cells were detected in LNs of all SIV-infected infants, while infected cells in the jejunum and colon were observed mainly in the rapid progressors and at lower numbers in the lung among all animals (Fig. 8G). Importantly, although the number of subjects was quite limited, the majority of SIV-infected cells in the rapid progressors were not CD3+ T cells but instead exhibited macrophage morphology and expressed CD163 (Fig. 8).
FIG 8.
Massive macrophage infection was observed in rapidly progressing SIV-infected pediatric macaques. Tissues obtained at necropsy were stained for SIV Env (KK41), CD163 (macrophages), and CD3 (T cells). Low- and high-magnification images, respectively, of jejunum (A and D), LN (B and E), and lung (C and F) are shown for animal JA96. Arrows and arrowheads indicate SIV-infected CD3+ T cells and CD163+ macrophages, respectively. Asterisks mark SIV+ CD3− and CD163− cells. (G) The numbers of SIV-infected CD3+ T cells, CD163+ macrophages, and CD3− CD163− cells per field were graphed for three rapid progressor (JA96, JL94, and JR01) and three conventional progressor (JF87, JD31, and KI13) infants.
DISCUSSION
SIV-infected newborn rhesus macaques rapidly progressed to AIDS after the acute stage of infection, unlike adult rhesus macaques, which transitioned from acute to chronic SIV infection before developing AIDS. Since macrophage infection and apoptosis in conjunction with increasing monocyte turnover and immigration to peripheral tissues occurred during terminal disease in adult SIV-infected rhesus macaques (28–30), we considered whether monocyte and macrophage functions in neonates and infants may affect the more rapid disease progression observed in the younger animals.
We first observed that naive non-SIV-infected neonate and infant macaques inherently exhibited a higher monocyte turnover rate than that observed in uninfected adult rhesus macaques, and by approximately 6 months of age, the monocyte turnover rate reached that observed in adults (Fig. 1). Increases in monocyte turnover rates also have been reported after experimental induction of inflammation, and the majority of monocytes immigrating to the site of inflammation were differentiating into macrophages (44–46). Experimental depletion of resident cells further demonstrated that circulating monocytes could reconstitute the tissue macrophages (47–51). It is thus plausible that the high monocyte turnover observed in infants may reflect a faster migration or trafficking of monocytes that will populate and differentiate into tissue macrophages. Another explanation could be that innate immune responses are accelerated to compensate for an immature adaptive immune system. Newborns experience an onslaught of new antigenic exposures after birth and the massive monocyte activation could be required to help control or limit these early infections. For example, the frequency of CD16+ CD14+ monocytes was higher in newborn macaques during the first week of life than at any other age, including older infants and adults (Fig. 1D and E). CD16+ CD14+ monocytes increased not only during SIV/HIV infection but also after other various microbiological infections (52–54). These monocytes possess activated and proinflammatory properties, such as high soluble CD163 production concomitant with increased turnover rates (55) and high CXCL10 expression in secondary lymphoid tissues (56). These activated monocytes also could be more susceptible to SIV infection, which may account for the more rapid propagation of virus during the acute phase. This agrees with previous reports demonstrating that monocytes and macrophages obtained from neonates were more susceptible to HIV infection in vitro (57, 58).
While a further elevated monocyte turnover rate from physiological baseline was observed in SIV-infected infant macaques soon after infection, monocyte turnover in newborn macaques was different from that in older infants during chronic phases of disease (Fig. 2 and 7). The consistently elevated monocyte turnover rate could be responsible for the rapid disease progression seen in newborns. It is important to delineate reasons why SIV-infected newborns more preferentially retained increased monocyte turnover over the older age group. Whereas increased monocyte turnover in acute phase of infection might be due to developmental needs of a growing neonate and maturing immune system, persistently high monocyte turnover in the chronic phase might induce AIDS-related diseases. If we can elucidate the role these cells play developmentally, as well as over the course of infection in infants, we may be able to target monocytes/macrophages for treatment. Therapeutic interventions could include initiation of ART at specific times based on the level of monocyte turnover (macrophage infection) or application of drugs that modify macrophages to become more resistant to HIV infection. Macrophages could also be targeted via drug delivery by using either liposomal bisphosphonates or nanoparticles carrying the drugs of interest.
We also observed that macrophages in various tissues produced high levels of SIV in the infant macaques (Fig. 8). Such massive infections in macrophages were also reported for pathogenic experimental models of infection with simian-human immunodeficiency virus (SHIV) (59), SIVsmE660 or SIVsmE543 in rapid progressors (60), and SIVmac251 after anti-CD4+ antibody treatment (61). In these studies, infected adult animals advanced to AIDS more rapidly, maintained high viral levels after reaching peak viremia, and failed to reduce viral levels for achieving a typical set point during chronic stage of infection, similar to what was observed in SIV-infected newborn macaques. Thus, these results further implicate macrophages as a site of viral replication, which may explain the persistent high plasma viral levels as well as contribute to pathogenesis in the setting of rapid disease progression in infant rhesus macaques.
A consequence of virus infection and the loss of the predominant population of CD163+ CD206+ macrophages in neonatal tissues may be acceleration of the onset of clinical signs associated with AIDS. One of the rapid progressor infants, JA96, exhibited a massive proportion of SIV-infected macrophages in all of tissues examined, and severe SIV-associated lesions were observed in brain (data not shown). Three of the SIV-infected rapid progressors also exhibited severe colitis. Since microbial translocation from damaged or dysregulated intestinal tissue could increase host susceptibility to additional pathogens, the loss of macrophages, in addition to declining CD4+ T cells caused by SIV infection, may cripple adaptive and innate immune responses, providing an environment more prone to opportunistic infections.
In summary, results from these studies identified a higher physiologic baseline monocyte turnover rate in the younger animals than in adults, which may reflect a less mature innate immune system and greater susceptibility to SIV pathogenesis. SIV infection of these younger animals induced further increases in monocyte turnover rates in conjunction with tissue macrophage infection and destruction. Continuously immigrating and differentiating monocytes to replace the damaged tissue macrophages thus may have promoted the continued increasing monocyte turnover and subsequent rapid disease progression. Taken together, these results advocate for monocyte and macrophage participation in the rapid disease progression of pediatric HIV/SIV infections and support studies to target innate immune mechanisms to reduce pathogenesis.
MATERIALS AND METHODS
Rhesus macaques and SIV infection.
Thirty-nine infant rhesus macaques specific pathogen free for SIV, type D simian retrovirus, herpes B virus, and simian T-cell leukemia virus type 1 from the Tulane National Primate Research Center were used in this study (Table 1). Newborn macaques were removed from their mothers, reared in an animal biosafety level 2 (ABSL2) nursery, and studied in accordance with the standards of the Guide for the Care and Use of Laboratory Animals (62) and with approval by the Institutional Animal Care and Use Committee of Tulane University. Animals were inoculated intravenously with 100 50% tissue culture infective doses (TCID50) of SIVmac251 within 24 h of birth (n = 15) or between 53 and 105 days of age (n = 4). Humane euthanasia was conducted at the scheduled time points and/or when warranted by manifestations of clinical AIDS or morbidity as indicated in Table 1. Neonates were defined as less than 1 month of age, and infants were defined as less than 1 year old.
BrdU/EdU injections.
To monitor cell turnover, thymidine analogues BrdU (Sigma-Aldrich) and EdU (Invitrogen) were used as described previously (39). Blood samples typically were collected 24 h after BrdU inoculations (or as indicated) to examine cell turnover of leukocytes. At the end of the study, tissues were collected 24 h or 48 h after BrdU or EdU inoculation at necropsy.
SIV quantification.
Plasma viral RNA loads were measured by a branched-DNA amplification assay (Siemens Diagnostics Clinical Laboratory) or real-time quantitative PCR targeting the SIVmac239 gag sequence (63).
Cell isolation from tissues.
Immune cells from intestinal tissues were isolated as described previously (14, 64), with some modifications. Briefly, excised sections of tissue were treated with 5 mM EDTA and Hanks' balanced salt solution to remove intestinal epithelial lymphocytes and then digested with collagenase type II (Sigma-Aldrich) in RPMI 1640 supplemented with 5% fetal bovine serum (FBS) to isolate leukocytes in the intestinal lamina propria. Leukocytes were purified by Percoll density gradient (35% and 60%) centrifugation (800 × g) for 20 min at 4°C. Immune cells from spleen and inguinal or axillary LNs were isolated by gentle mincing and digestion with collagenase type IV (Worthington) in RPMI 1640 supplemented with 5% FBS, followed by removal of residual red blood cells by treatment with ACK lysing buffer (Lonza).
Flow cytometry.
Blood and tissue cells were stained for 10-color flow cytometric analysis, and the following monoclonal antibodies (MAbs) were used: CD3-Alexa Fluor 700 (AF700) and -Pacific Blue (PB) (SP34-2), CD4-peridinin chlorophyll protein (PerCP)-Cy5.5 (L200), CD8-V500 (SK1), CD11b-AF700 (clone), CD14-PB (M5E2), CD16-allophycocyanin (APC)-H7 (3G8), CD20-APC-H7 (2H7), CD95-APC (DX2), CD206-APC (19.2), HLA-DR–phycoerythrin (PE)–Cy7 (L243), BrdU-fluorescein isothiocyanate (FITC), CD20-electron-coupled dye (ECD) (B9E9), CD28-ECD (28.1), and CD163-PE (Mac2-158). The MAbs were obtained from BD Biosciences, Beckman Coulter, and Trillium. Surface-stained cells were permeabilized by sequential incubations with BD Cytofix/Cytoperm buffer (BD Biosciences) and DNase treated at 37°C for 1 h, followed by staining with FITC anti-BrdU. Cells were resuspended in phosphate-buffered saline (PBS) containing 1% formaldehyde and acquired on an LSR II, LSR Fortessa, or FACS Aria (BD Biosciences). The data were analyzed using FlowJo software (TreeStar).
Immunohistochemistry and confocal microscopy imaging.
Formalin-fixed, paraffin-embedded tissues from animals were sectioned at a thickness of 7 μm. The following primary antibodies (Abs) were used: anti-CD163 mouse monoclonal antibody (10D6; Novocastra), anti-CD206 rabbit polyclonal antibody (Sigma-Aldrich), and anti-CD3 rabbit polyclonal antibody (Dako). Anti-BrdU rat monoclonal antibody (BU1/75; Novus) was used for staining the tissue sections, followed by incubation with AF488-, AF568-, or AF633-conjugated secondary antibodies (1:1,000; Invitrogen). To maximize the SIV signal, a tyramide signal amplification (TSA) kit (Invitrogen) was used, followed by staining with anti-SIV gp160/gp41 mouse monoclonal antibody (KK41; NIH AIDS Reagent Program). Apoptotic cells were detected with an ApopTag Plus Fluorescein In Situ Apoptosis Detection kit (Millipore) for terminal deoxynucleotidyltransferase dUTP nick end labeling (TUNEL). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope (Leica Microsystems). Adobe Photoshop (Adobe Systems) was used to assign colors to the three channels collected (AF633 [blue], AF568 [red], and AF488 or fluorescein [green]). NIH Image (version 1.62; NIH) was used to count cells in images. Cells were manually counted in five randomly selected fields with an area of 0.14 mm2 each per tissue section, and the mean numbers of cells were reported.
Statistical analysis.
The Mann-Whitney test was used to compare two unmatched treatment groups. The Kruskal-Wallis test was applied to compare more than two unpaired groups, followed by Dunn's posttest. Spearman nonparametric analysis was applied for assessing correlations. GraphPad Prism 6.0 software for Mac (GraphPad, San Diego, CA) was used to analyze data and prepare figures. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
Contributions to this work were as follows: C.S., A.H., and M.J.K. designed the studies; C.S., A.H., X.W., and R.S.V. participated in the execution of experiments; C.S., A.H., X.A.A., H.W., and M.J.K. analyzed the data; C.S., A.H., K.M.M., X.A.A., H.W., K.M., W.-K.K., E.S.D., and M.J.K. interpreted the results; and C.S., K.M.M., E.S.D., and M.J.K. prepared the manuscript.
We acknowledge the following grants from the NIH: R01AI097059, R21AI091501, R01HL125054, R21AI116198, and R21MH108458. The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIH: anti-SIVmac251 gp41 monoclonal antibody (KK41) from Karen Kent and Caroline Powell.
We claim no conflicts of interest.
REFERENCES
- 1.Forbes JC, Alimenti AM, Singer J, Brophy JC, Bitnun A, Samson LM, Money DM, Lee TC, Lapointe ND, Read SE. 2012. A national review of vertical HIV transmission. AIDS 26:757–763. doi: 10.1097/QAD.0b013e328350995c. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2014. Global update on the health sector response to HIV, 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/progressreports/update2014/en/ Accessed 5 February 2017. [Google Scholar]
- 3.Locks LM, Manji KP, Kupka R, Liu E, Kisenge R, McDonald CM, Aboud S, Wang M, Fawzi WW, Duggan CP. 2017. High burden of morbidity and mortality but not growth failure in infants exposed to but uninfected with human immunodeficiency virus in Tanzania. J Pediatr 180:191–199.e192. doi: 10.1016/j.jpeds.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. 2004. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 5.Goulder PJ, Lewin SR, Leitman EM. 2016. Paediatric HIV infection: the potential for cure. Nat Rev Immunol 16:259–271. doi: 10.1038/nri.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tovo PA, de Martino M, Gabiano C, Cappello N, D'Elia R, Loy A, Plebani A, Zuccotti GV, Dallacasa P, Ferraris G, Caselli D, Fundaró C, D'Argenio P, Principi N, Stegagno M. 1992. Prognostic factors and survival in children with perinatal HIV-1 infection. Lancet 339:1249–1253. [DOI] [PubMed] [Google Scholar]
- 7.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 8.Veazey R, Lackner A. 2003. The mucosal immune system and HIV-1 infection. AIDS Rev 5:245–252. [PubMed] [Google Scholar]
- 9.Epple HJ, Zeitz M. 2012. HIV infection and the intestinal mucosal barrier. Ann N Y Acad Sci 1258:19–24. doi: 10.1111/j.1749-6632.2012.06512.x. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. 2000. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet 355:1131–1137. [PubMed] [Google Scholar]
- 11.Bunders MJ, van der Loos CM, Klarenbeek PL, van Hamme JL, Boer K, Wilde JC, de Vries N, van Lier RA, Kootstra N, Pals ST, Kuijpers TW. 2012. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood 120:4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty R. 2005. HIV-1 infection in children: a clinical and immunologic overview. Curr HIV Res 3:31–41. doi: 10.2174/1570162052773022. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Xu H, Pahar B, Alvarez X, Green LC, Dufour J, Moroney-Rasmussen T, Lackner AA, Veazey RS. 2010. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood 116:4168–4174. doi: 10.1182/blood-2010-03-273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veazey RS, Lifson JD, Pandrea I, Purcell J, Piatak M, Lackner AA. 2003. Simian immunodeficiency virus infection in neonatal macaques. J Virol 77:8783–8792. doi: 10.1128/JVI.77.16.8783-8792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torow N, Yu K, Hassani K, Freitag J, Schulz O, Basic M, Brennecke A, Sparwasser T, Wagner N, Bleich A, Lochner M, Weiss S, Forster R, Pabst O, Hornef MW. 2015. Active suppression of intestinal CD4(+)TCRalphabeta(+) T-lymphocyte maturation during the postnatal period. Nat Commun 6:7725. doi: 10.1038/ncomms8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, Granot T, Griesemer A, Lerner H, Kato T, Farber DL. 2016. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med 22:72–77. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. 1998. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica 83:197–203. [PubMed] [Google Scholar]
- 18.Peoples JD, Cheung S, Nesin M, Lin H, Tatad AM, Hoang D, Perlman JM, Cunningham-Rundles S. 2009. Neonatal cord blood subsets and cytokine response to bacterial antigens. Am J Perinatol 26:647–657. doi: 10.1055/s-0029-1220788. [DOI] [PubMed] [Google Scholar]
- 19.Waecker NJ Jr, Ascher DP, Robb ML, Moriarty R, Krober M, Rickman WJ, Butzin CA, Fischer GW. 1993. Age-adjusted CD4+ lymphocyte parameters in healthy children at risk for infection with the human immunodeficiency virus. The Military Pediatric HIV Consortium. Clin Infect Dis 17:123–125. [DOI] [PubMed] [Google Scholar]
- 20.Gesner M, Papaevangelou V, Kim M, Chen SH, Moore T, Krasinski K, Borkowsky W. 1994. Alteration in the proportion of CD4 T lymphocytes in a subgroup of human immunodeficiency virus-exposed-uninfected children. Pediatrics 93:624–630. [PubMed] [Google Scholar]
- 21.The European Collaborative Study. 1992. Age-related standards for T lymphocyte subsets based on uninfected children born to human immunodeficiency virus 1-infected women. Pediatr Infect Dis J 11:1018–1026. doi: 10.1097/00006454-199211120-00006. [DOI] [PubMed] [Google Scholar]
- 22.Plaeger-Marshall S, Hultin P, Bertolli J, O'Rourke S, Kobayashi R, Kobayashi AL, Giorgi JV, Bryson Y, Stiehm ER. 1993. Activation and differentiation antigens on T cells of healthy, at-risk, and HIV-infected children. J Acquir Immune Defic Syndr 6:984–993. [PubMed] [Google Scholar]
- 23.Rich KC, Brambilla D, Pitt J, Moye J, Cooper E, Hillyer G, Mendez H, Fowler MG, Landay A. 1997. Lymphocyte phenotyping in infants: maturation of lymphocyte subpopulations and the effects of HIV infection. Clin Immunol Immunopathol 85:273–281. doi: 10.1006/clin.1997.4439. [DOI] [PubMed] [Google Scholar]
- 24.Tobin NH, Aldrovandi GM. 2013. Immunology of pediatric HIV infection. Immunol Rev 254:143–169. doi: 10.1111/imr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattentau QJ, Stevenson M. 2016. Macrophages and HIV-1: an unhealthy constellation. Cell Host Microbe 19:304–310. doi: 10.1016/j.chom.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiNapoli SR, Hirsch VM, Brenchley JM. 2016. Macrophages in progressive human immunodeficiency virus/simian immunodeficiency virus infections. J Virol 90:7596–7606. doi: 10.1128/JVI.00672-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda MJ. 2010. Macrophages: do they impact AIDS progression more than CD4 T cells? J Leukoc Biol 87:569–573. doi: 10.1189/jlb.0909626. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. 2009. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Y, Sugimoto C, Liu DX, Midkiff CC, Alvarez X, Lackner AA, Kim WK, Didier ES, Kuroda MJ. 2015. Increased monocyte turnover is associated with interstitial macrophage accumulation and pulmonary tissue damage in SIV-infected rhesus macaques. J Leukoc Biol 97:1147–1153. doi: 10.1189/jlb.4A0914-441R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Y, Sugimoto C, Arainga M, Midkiff CC, Liu DX, Alvarez X, Lackner AA, Kim WK, Didier ES, Kuroda MJ. 2015. Preferential destruction of interstitial macrophages over alveolar macrophages as a cause of pulmonary disease in simian immunodeficiency virus-infected rhesus macaques. J Immunol 195:4884–4891. doi: 10.4049/jimmunol.1501194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. 2010. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams K, Burdo TH. 2012. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol 7:363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 33.Crowe S, Zhu T, Muller WA. 2003. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol 74:635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto C, Hasegawa A, Saito Y, Fukuyo Y, Chiu KB, Cai Y, Breed MW, Mori K, Roy CJ, Lackner AA, Kim WK, Didier ES, Kuroda MJ. 2015. Differentiation kinetics of blood monocytes and dendritic cells in macaques: insights to understanding human myeloid cell development. J Immunol 195:1774–1781. doi: 10.4049/jimmunol.1500522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim WK, Sun Y, Do H, Autissier P, Halpern EF, Piatak M, Lifson JD, Burdo TH, McGrath MS, Williams K. 2010. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol 87:557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. 2009. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol 130:338–346. doi: 10.1016/j.clim.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Nockher WA, Scherberich JE. 1998. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun 66:2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holder GE, McGary CM, Johnson EM, Zheng R, John VT, Sugimoto C, Kuroda MJ, Kim WK. 2014. Expression of the mannose receptor CD206 in HIV and SIV encephalitis: a phenotypic switch of brain perivascular macrophages with virus infection. J Neuroimmune Pharmacol 9:716–726. doi: 10.1007/s11481-014-9564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. 2014. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol 192:2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K. 2013. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev 22:985–997. doi: 10.1089/scd.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyama WM, Randolph GJ, Unanue ER. 2015. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 212:1497–1512. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies LC, Jenkins SJ, Allen JE, Taylor PR. 2013. Tissue-resident macrophages. Nat Immunol 14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. 1972. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ 46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 45.Goto H, Inaba M, Kobayashi K, Imanishi Y, Kumeda Y, Inui K, Okada F, Nishizawa Y. 2003. Successful treatment of multicentric reticulohistiocytosis with alendronate: evidence for a direct effect of bisphosphonate on histiocytes. Arthritis Rheum 48:3538–3541. doi: 10.1002/art.11362. [DOI] [PubMed] [Google Scholar]
- 46.Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 47.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. 2006. Langerhans cells arise from monocytes in vivo. Nat Immunol 7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Brück W, Priller J, Prinz M. 2007. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 49.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. 2010. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One 5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. 2009. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 51.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. 2009. Origin of the lamina propria dendritic cell network. Immunity 31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aktas Cetin E, Pur Ozyigit L, Gelmez YM, Cakir E, Gedik AH, Deniz G. 2017. CD163 levels, pro- and anti-inflammatory cytokine secretion of monocytes in children with pulmonary tuberculosis. Pediatr Pulmonol 52:675–683. doi: 10.1002/ppul.23617. [DOI] [PubMed] [Google Scholar]
- 53.Ren X, Mou W, Su C, Chen X, Zhang H, Cao B, Li X, Wu D, Ni X, Gui J, Gong C. 2017. Increase in peripheral blood intermediate monocytes is associated with the development of recent-onset type 1 diabetes mellitus in children. Int J Biol Sci 13:209–218. doi: 10.7150/ijbs.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singla M, Kar M, Sethi T, Kabra SK, Lodha R, Chandele A, Medigeshi GR. 2016. Correction: immune response to dengue virus infection in pediatric patients in New Delhi, India—association of viremia, inflammatory mediators and monocytes with disease severity. PLoS Negl Trop Dis 10:e0004642. doi: 10.1371/journal.pntd.0004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Micci L, Alvarez X, Iriele RI, Ortiz AM, Ryan ES, McGary CS, Deleage C, McAtee BB, He T, Apetrei C, Easley K, Pahwa S, Collman RG, Derdeyn CA, Davenport MP, Estes JD, Silvestri G, Lackner AA, Paiardini M. 2014. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog 10:e1004467. doi: 10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujino M, Sato H, Okamura T, Uda A, Takeda S, Ahmed N, Shichino S, Shiino T, Saito Y, Watanabe S, Sugimoto C, Kuroda M, Ato M, Nagai Y, Izumo S, Matsushima K, Miyazawa M, Ansari AA, Villinger F, Mori K. 19 April 2017. Simian immunodeficiency virus targeting of CXCR3(+) CD4(+) T cells in secondary lymphoid organs is associated with robust CXCL10 expression in monocyte/macrophage subsets. J Virol doi: 10.1128/JVI.00439-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sperduto AR, Bryson YJ, Chen IS. 1993. Increased susceptibility of neonatal monocyte/macrophages to HIV-1 infection. AIDS Res Hum Retroviruses 9:1277–1285. doi: 10.1089/aid.1993.9.1277. [DOI] [PubMed] [Google Scholar]
- 58.Ho WZ, Lioy J, Song L, Cutilli JR, Polin RA, Douglas SD. 1992. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol 66:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A 98:658–663. doi: 10.1073/pnas.98.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown CR, Czapiga M, Kabat J, Dang Q, Ourmanov I, Nishimura Y, Martin MA, Hirsch VM. 2007. Unique pathology in simian immunodeficiency virus-infected rapid progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J Virol 81:5594–5606. doi: 10.1128/JVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, Sternberg L, Lawson B, Carnathan PM, Cramer EM, Engram JC, Little DM, Ryzhova E, Gonzalez-Scarano F, Paiardini M, Ansari AA, Ratcliffe S, Else JG, Brenchley JM, Collman RG, Estes JD, Derdeyn CA, Silvestri G. 2011. Depletion of CD4+ T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest 121:4433–4445. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 63.Monjure CJ, Tatum CD, Panganiban AT, Arainga M, Traina-Dorge V, Marx PA, Didier ES. 2014. Optimization of PCR for quantification of simian immunodeficiency virus genomic RNA in plasma of rhesus macaques (Macaca mulatta) using armored RNA. J Med Primatol 43:31–43. doi: 10.1111/jmp.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, Johnson RP, Lackner AA. 1997. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin Immunol Immunopathol 82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]