Abstract
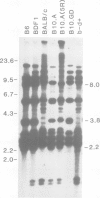
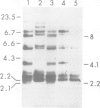
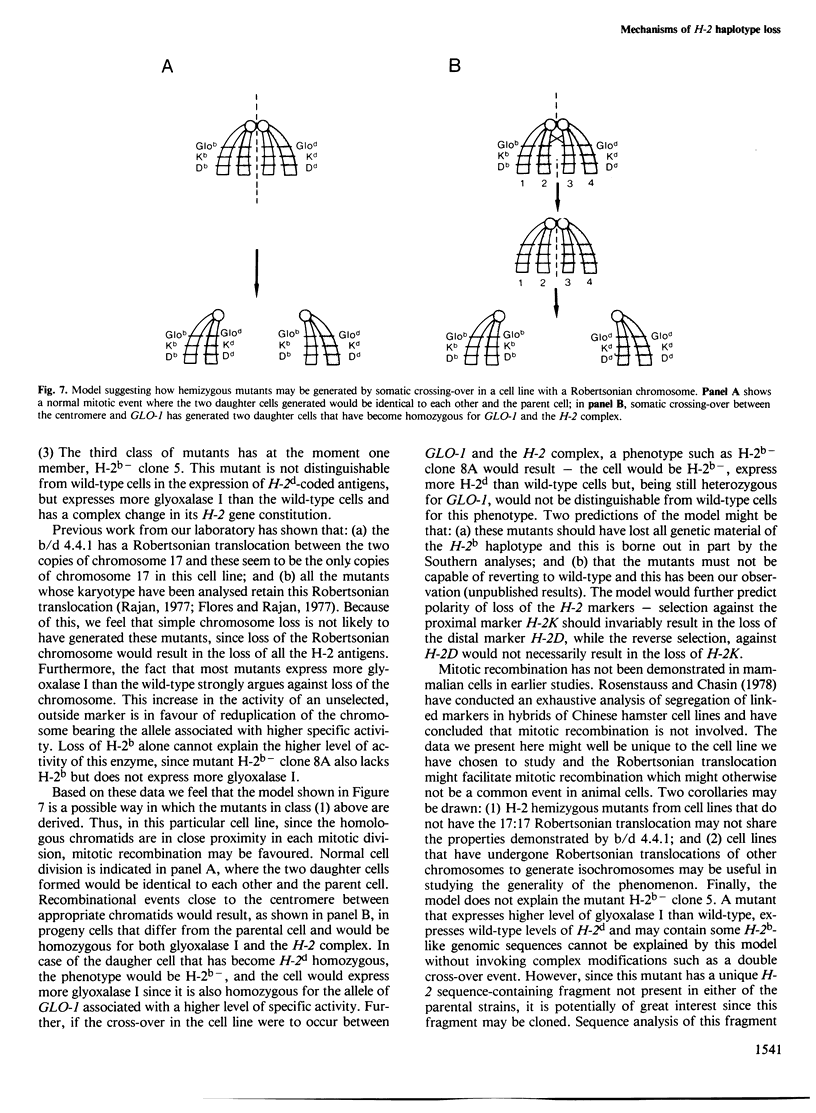
Variants that no longer express an entire H-2 haplotype were readily isolated, by immunoselection with antisera directed against the haplotype, from an H-2b/H-2d heterozygous Friend leukemia cell line carrying a Robertsonian translocation of the chromosomes bearing the H-2 genetic region. These variants can be denoted as being of the phenotype H-2b- H-2d+ or H-2b+ H-2d-. Some of the H-2b- H-2d+ variants: (1) lack the restriction enzyme fragments characteristic of the missing H-2b haplotype, as assessed by Southern blot analysis; (2) express more cell surface H-2d antigens than wild-type cells, as assessed by flow microfluorimetry; and (3) appear to have become homozygous for the more active H-2d-linked allele at the Glyoxalase I locus. These variants thus seem to have lost genetic material corresponding to the H-2b haplotype and may have gained genetic material corresponding to the H-2d haplotype. These results are consistent with the possibility that these variants were generated by mitotic recombination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester M. V., Norton S. J. The isolation and characterization of mouse liver glyoxalase I. Biochim Biophys Acta. 1975 May 23;391(1):212–221. doi: 10.1016/0005-2744(75)90168-0. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Evans G. A., Flaherty L., Seidman J. G. H-2-like genes in the Tla region of mouse chromosome 17. Nature. 1982 Jan 14;295(5845):168–170. doi: 10.1038/295168a0. [DOI] [PubMed] [Google Scholar]
- Meo T., Douglas T., Rijnbeek A. M. Glyoxalase I polymorphism in the mouse: a new genetic marker linked to H-2. Science. 1977 Oct 21;198(4314):311–313. doi: 10.1126/science.910130. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Bach F. H., Ploegh H. L., Strominger J. L., Kavathas P., DeMars R. Use of HLA loss mutants to analyse the structure of the human major histocompatibility complex. Nature. 1982 Apr 1;296(5856):454–456. doi: 10.1038/296454a0. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Nathenson S. G., Leinwand L. A. Mapping class I gene sequences in the major histocompatibility complex. Nature. 1982 Jul 22;298(5872):382–385. doi: 10.1038/298382a0. [DOI] [PubMed] [Google Scholar]
- Pious D., Krangel M. S., Dixon L. L., Parham P., Strominger J. L. HLA antigen structural gene mutants selected with an allospecific monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7832–7836. doi: 10.1073/pnas.79.24.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter T. A., Hansen T. H., Habbersett R., Ozato K., Ahmed A. Flow microfluorometric analysis of H-2L expression. J Immunol. 1981 Aug;127(2):580–584. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Separation of linked markers in Chinese hamster cell hybrids: mitotic recombination is not involved. Genetics. 1978 Dec;90(4):735–760. doi: 10.1093/genetics/90.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]