Abstract
Background
There are little data available regarding pattern of first metastases in resected MM as well as the response of advanced MM to cytotoxic therapy.
Patients and Methods
A retrospective, single-institution cohort was assembled of all patients with advanced/unresectable MM between 1995 and 2012 who received systemic therapy with available imaging (N=81). Responses to first- and second-line systemic therapy were assessed using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The relationship between response, OS, and clinical covariates was investigated using Cox proportional hazards regression.
Results
Primary sites included anorectal (N=31, 38%), vulvovaginal (N=28, 35%), head and neck (N=21, 26%), and gallbladder (N=1, 1%) mucosa. Seven percent of patients first relapsed in the brain. Cytotoxic therapy represented 82% and 51% of first- and second-line regimens. Best response achieved in the first-line setting was similar for single-agent (10%; 95% CI: 1–32%) and combination alkylator therapy (8%; 95% CI: 2–21%). Median OS from 1st-line treatment was 10.3 months (95% CI 8.7–13.9 months). Patients with elevated LDH (HR 1.87, 95% CI: 1.10–3.19, p=0.020) and ECOG performance status 1–2 (HR 1.69, 95% CI: 1.05–2.72, p=0.030) had a higher risk of death, while patients with 12-week objective responses had a lower risk of death (HR: 0.12, 95% CI: 0.04–0.41, p<0.001).
Conclusion
Cytotoxic systemic therapy has modest activity in advanced/unresectable MM, belying its adjuvant benefit. Patients whose tumors have an objective response to therapy have a lower chance of death. Brain imaging should be considered in routine surveillance.
Keywords: Mucosal melanoma, cytotoxic therapy, metastasis, prognosis
Introduction
Malignant melanoma is a heterogeneous group of melanocytic neoplasms diagnosed in approximately 76,000 people in the United States yearly [1]. Mucosal melanomas (MM) comprise 1–2% of all melanomas in the US and arise from non-hair bearing mucosal surfaces of the body, most commonly the head and neck, anorectal, or vulvovaginal regions [2]. MM is clinically and genetically distinct from cutaneous melanoma [3, 4]. Patients diagnosed with MM tend to be older than those with cutaneous or uveal primary sites, and their stage-matched prognosis is poorer [5, 6]. A retrospective review of survival from the time of metastasis in 2920 patients with melanoma of various primary sites demonstrated that those with MM lived a median (95% confidence interval (CI)) of 9.1 (7.6 – 9.8) months, versus 13.4 (11.6–15.6) and 11.7 (11.2 – 12.6) months for those with uveal and cutaneous disease, respectively [6]. It is not clear whether clinical factors such as site of first metastasis or a lower response to systemic therapy influence this poorer prognosis.
The most rigorous MM therapy study to date was a large, single-center randomized Phase 2 trial that enrolled 189 patients with resected MM in a 1:1:1 ratio to observation, adjuvant interferon alpha, or adjuvant cisplatin plus temozolomide [7]. Patients receiving adjuvant cisplatin plus temozolomide or high-dose interferon had a significant improvement in overall survival (48.7 vs 40.4 vs 21.2 months, respectively; p<0.01 for both comparisons). All patients on the observation arm recurred within 2 years, and seventy-five percent recurred either regionally or in a distant site [7].
Given the significant benefit of adjuvant therapy with cytotoxic agents and interferon, there is a need to document the activity of cytotoxic therapy in the advanced setting. To our knowledge, there are only four published retrospective series reporting response of MM to cytotoxic or biochemotherapy. The largest included 28 patients receiving dacarbazine-based chemotherapy and reported a similar objective response rate (ORR) between non-cutaneous and cutaneous melanoma (CM) of 20 and 30%, respectively [8]. The other studies reported responses in 7/13 (54%) head and neck MM, 8/18 (44%) of anorectal MM, and 4/11 (36%) of vulvovaginal MM with biochemotherapy, which combines cytotoxic agents such as cisplatin, vinblastine, and dacarbazine (CVD) with immunomodulatory therapy such as interferon alpha and/or interleukin (IL)-2 [9–11].
Considering the poor prognosis for patients with MM, the magnitude of OS benefit with cytotoxic therapy, and the relative paucity of published data, we sought to assess the patterns of first metastasis, response rates, and overall survival for patients with MM treated with any systemic agents at our institution.
Methods
After obtaining IRB approval, we used institutional databases to identify patients who were diagnosed with metastatic or unresectable MM between 1/1/1995 and 12/31/2012, received ≥1 non-adjuvant systemic therapy, and had CT, MRI, or PET imaging available for radiological evaluation.
We collected standard demographic data, such as age, gender, race, ECOG performance status, tumor stage, prior receipt of adjuvant therapy, and systemic therapies received in the first- and second-line settings for advanced or unresectable disease. Patients were classified by primary disease site: anorectal, vulvovaginal, head/neck, and gallbladder.
CT and/or MRIs were collected at baseline and during the course of treatment for advanced or unresectable disease. Initial sites of metastatic disease were identified using radiographic or pathologic reports and categorized by organ system. Multiple nodules in an organ were counted only once. Responses to first- and second-line regimens were determined using RECIST 1.1 by radiologists (MB and RL) blinded to treatment.
Patients with metastatic disease were staged using American Joint Committee on Cancer 7th edition guidelines [12] for cutaneous melanoma (M1a, M1b or M1c). Therapies were categorized into the following groups: single-agent alkylator (e.g., temozolomide or dacarbazine); combination alkylator (e.g., cisplatin, vinblastine and temozolomide [CVT]); non-alkylating cytotoxic; immunotherapy; biochemotherapy; targeted agents; and other.
Statistical Methods
Patient characteristics were summarized using medians and ranges for continuous variables and frequencies and percent for categorical variables. Differences between site and adjuvant therapy rates were tested using Fisher’s exact test. Twelve week response rate was defined as partial (PR) or complete (CR) response using RECIST 1.1 by 12 weeks after the commencement of line 1 systemic therapy. For all response proportions, percent of response was provided along with exact 95% confidence intervals.
Overall best response (BR) was defined as PR or CR using RECIST 1.1 during any point of the chemotherapy regimen. BR was measured separately for each line of therapy. Patients without scans during the duration of therapy were excluded from the analysis. Twenty-four week disease control rate (DCR) was defined as RECIST 1.1 PR, CR, or stable disease (SD) at or after 24 weeks of treatment in all patients evaluable for response. If a patient stopped or changed regimens before 24 weeks or did not have a scan available, they were considered not controlled.
Overall Survival (OS) was defined as the time interval between chemotherapy line 1 commencement and death or last follow up. Patients alive at last follow up were censored. Factors associated with overall survival were evaluated using Cox proportional hazards regression and twelve week response was modeled as a time dependent covariate. OS stratified by response rate was plotted by Kaplan Meier methods landmarked at 12 weeks. P values <0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R 3.1.1 (The R Foundation).
Results
Patient Characteristics
Patient characteristics and demographics are shown in Table 1. Overall, 81 patients met the inclusion criteria. The median age at diagnosis was 63 years (range: 33–89 years). Seventy-three percent of patients were female (59/81) and 82% were Caucasian (66/81). Thirty-eight percent had disease arising from the anorectal (31/81) mucosa, 26% from the head and neck (21/81), 35% from the vulvovaginal mucosa (28/81), and 1% from the gallbladder (1/81). Eighty-four percent underwent resection of the primary disease (68/81).
Table 1.
Patient Characteristics
| Site | ||||||
|---|---|---|---|---|---|---|
| All (N=81) |
Anorectal (N=31) |
Head & Neck (N=21) |
Vulvovaginal (N=28) |
|||
| Gender | Male | 22 (27.2) | 8 (25.8) | 14 (66.7) | 0 (0) | |
| Female | 59 (72.8) | 23 (74.2) | 7 (33.3) | 28 (100) | ||
| Race | White | 66 (81.5) | 25 (80.6) | 16 (76.2) | 24 (85.7) | |
| Black | 6 (7.4) | 2 (6.5) | 2 (9.5) | 2 (7.1) | ||
| Asian | 8 (9.9) | 4 (12.9) | 3 (14.3) | 1 (3.6) | ||
| Unknown | 1 (1.2) | 0 (0) | 0 (0) | 1 (3.6) | ||
| Age at Diagnosis (years) | Median (range) | 62.6 (32.9–89.4) | 61.1 (35.7–82.1) | 63.2 (32.9–81.9) | 65.1 (39.9–89.4) | |
| Mutation Status | ||||||
| KIT | Wild Type | 34 (42) | 14 (45.2) | 10 (47.6) | 10 (35.7) | |
| Mutant | 9 (11.1) | 2 (6.5) | 1 (4.8) | 6 (21.4) | ||
| Unknown | 38 (46.9) | 15 (48.4) | 10 (47.6) | 12 (42.9) | ||
| BRAF | Wild Type | 35 (43.2) | 12 (38.7) | 9 (42.9) | 14 (50) | |
| Mutant | 3 (3.7) | 2 (6.5) | 1 (4.8) | 0 (0) | ||
| Unknown | 43 (53.1) | 17 (54.8) | 11 (52.4) | 14 (50) | ||
| NRAS | Wild Type | 31 (38.3) | 11 (35.5) | 9 (42.9) | 11 (39.3) | |
| Mutant | 5 (6.2) | 1 (3.2) | 1 (4.8) | 3 (10.7) | ||
| Unknown | 45 (55.6) | 19 (61.3) | 11 (52.4) | 14 (50) | ||
| Surgery | No | 13 (16) | 3 (9.7) | 4 (19) | 6 (21.4) | |
| Yes | 68 (84) | 28 (90.3) | 17 (81) | 22 (78.6) | ||
| Adjuvant Treatment | ||||||
| Chemotherapy | No | 47 (58) | 15 (48.4) | 15 (71.4) | 16 (57.1) | |
| Yes | 21 (25.9) | 13 (41.9) | 2 (9.5) | 6 (21.4) | ||
| n/a | 13 (16) | 3 (9.7) | 4 (19) | 6 (21.4) | ||
| Radiation Therapy | No | 43 (53.1) | 27 (87.1) | 1 (4.8) | 14 (50) | |
| Yes | 25 (30.9) | 1 (3.2) | 16 (76.2) | 8 (28.6) | ||
| n/a | 13 (16) | 3 (9.7) | 4 (19) | 6 (21.4) | ||
| At Treatment Start | ||||||
| Stage | M1a/M1b | 16 (19.8) | 8 (25.8) | 3 (14.3) | 5 (17.9) | |
| M1c | 52 (64.2) | 19 (61.3) | 15 (71.4) | 17 (60.7) | ||
| M0 | 13 (16) | 4 (12.9) | 3 (14.3) | 6 (21.4) | ||
| ECOG PS | 0 | 51 (63) | 23 (74.2) | 11 (52.4) | 17 (60.7) | |
| 1 | 24 (29.6) | 6 (19.4) | 9 (42.9) | 8 (28.6) | ||
| 2 | 5 (6.2) | 2 (6.5) | 1 (4.8) | 2 (7.1) | ||
| Unknown | 1 (1.2) | 0 (0) | 0 (0) | 1 (3.6) | ||
| LDH Status | Normal | 36 (44.4) | 14 (45.2) | 8 (38.1) | 14 (50) | |
| Elevated | 27 (33.3) | 12 (38.7) | 8 (38.1) | 7 (25) | ||
| Unknown | 18 (22.2) | 5 (16.1) | 5 (23.8) | 7 (25) | ||
| Chemotherapy | ||||||
| Line 1 Duration (weeks) | Median (range) | 9 (0.9–65.2) | 10.5 (3.6–43.1) | 7.7 (3–24.9) | 8.9 (0.9–65.2) | |
| Line 2 Duration (weeks) | Median (range) | 9 (0.3–266.7) | 8 (3–57.3) | 9.8 (8.8–37.2) | 11.3 (0.3–266.7) | |
| Follow up Time (months) | Median (range) | 10.3 (0.5–90.8) | 12.4 (5.1–90.8) | 7.3 (1.8–81.1) | 10.6 (0.5–77.5) | |
This table excludes 1 patient with gallbladder primary.
Mutation status of KIT, BRAF, and NRAS genes was available for a subset of tumors. Nine of 43 (21%) were found to harbor KIT mutations, with 8 affecting exon 11 (6 L576P, 1 V560D, 1 deletion) and 1 affecting exon 13 (V654A). Three of 38 (8%) harbored BRAF mutations, with 2 D594 missense mutations and 1 T599I mutation identified. Five of 36 (14%) harbored NRAS mutations, with 3 Q61 mutations and 2 G12D mutations observed.
Pattern of Initial Metastases
At time of first non-adjuvant systemic treatment, 84% of patients had metastatic disease identified on imaging and 16% had locally advanced unresectable disease. LDH was elevated in 33% of cases.
At the initial diagnosis of metastatic disease, 143 organ sites were involved (Figure 1). Forty-eight percent of patients had metastasis limited to 1 organ site. The most common sites of initial metastasis were the liver (57%), lung (41%), non-regional LN (38%), and soft tissue (22%). Bone metastases were observed in 9 cases (12%): six from head and neck, 3 from anorectal, and none from vulvovaginal MM. Brain metastases were relatively uncommon, but were observed as an initial metastatic site in 5 cases (7%), including 2 vulvovaginal (1 vulvar, 1 vaginal) melanomas and 1 each from rectal, nasal cavity, and gallbladder.
Figure 1.
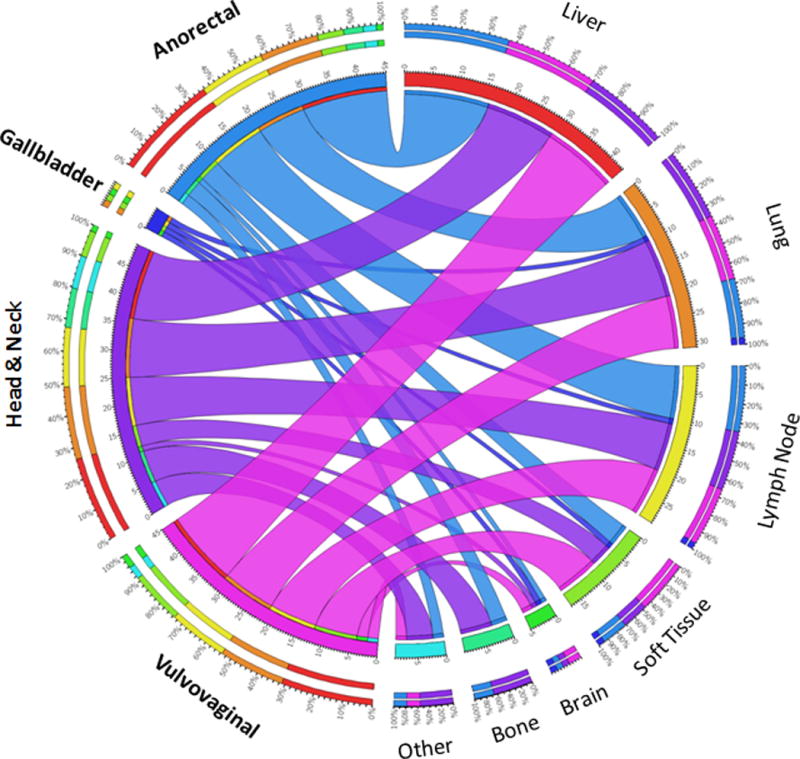
Circos plot of all initial metastatic sites (n=143) for 76 patients. The 143 sites are broken down by primary site on the left half of the circle and the metastatic organ on the right half, with the segments representing the relative abundance of metastases by primary site and destination. Ribbons connect the primary site with the metastatic organ system, with the color corresponding to the primary site associated with that colored segment (e.g. anorectal, blue) and the width denoting the number of cases that metastasized to that site (e.g. anorectal spread to liver, n=16). Relative percentages of spread are represented on the outer segments; for example, 36% of anorectal metastases were to the liver (red, 11 o’clock) and 67% of bone metastases originated from the head and neck (purple, near 6 o’clock).
Treatment
Seventy-four and 43 patients were evaluable for response to first- and second-line systemic therapy, respectively (Table 2). Sixty-one (82%) first-line regimens and 22 (51%) second-line regimens included cytotoxic agents. Of these, 38 (62%) and 15 (68%), respectively, were combination alkylator regimens; 20 (33%) and 2 (5%), respectively, were single agent alkylators; and 3 (5%) and 5 (12%), respectively, were non-alkylator regimens
Table 2.
Systemic Therapy Regimens Administered
| Chemotherapy Line | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | 1 | 2 | ||||||
| N (%) | N (%) | N (%) | ||||||
| Cytotoxic Therapy | ||||||||
| Single Agent Alkylator | Dacarbazine | 4 | (3.4) | 4 | (5.4) | 0 | (0.0) | |
| Temozolomide | 18 | (15.4) | 16 | (21.6) | 2 | (4.7) | ||
| Combination Alkylator | Temozolomide + Cisplatin +/− other agent(s) | 6 | (5.1) | 4 | (5.4) | 2 | (4.7) | |
| CVT/CVD | 34 | (29.1) | 23 | (31.1) | 11 | (25.6) | ||
| Other Alkylator Combination | 3 | (2.6) | 2 | (2.7) | 1 | (2.3) | ||
| Temozolomide + thalidomide | 10 | (8.5) | 9 | (12.2) | 1 | (2.3) | ||
| Other Cytotoxic | Anthracycline based | 2 | (1.7) | 1 | (1.4) | 1 | (2.3) | |
| Carboplatin + paclitaxel | 4 | (3.4) | 0 | (0.0) | 4 | (9.3) | ||
| FOLFIRI + flavopiridol | 2 | (1.7) | 2 | (2.7) | 0 | (0.0) | ||
| Immune Therapy | ||||||||
| Immune checkpoint inhibitor | CD137 Agonist | 1 | (0.9) | 0 | (0.0) | 1 | (2.3) | |
| Ipilimumab | 10 | (8.5) | 4 | (5.4) | 6 | (14.0) | ||
| Nivolumab | 2 | (1.7) | 0 | (0.0) | 2 | (4.7) | ||
| Pembrolizumab | 1 | (0.9) | 0 | (0.0) | 1 | (2.3) | ||
| Biochemotherapy | CVT/CVD + IL-2, IFNa | 5 | (4.3) | 2 | (2.7) | 3 | (7.0) | |
| Other | 1 | (0.9) | 1 | (1.4) | 0 | (0.0) | ||
| Targeted treatment | Imatinib | 6 | (5.1) | 3 | (4.1) | 3 | (7.0) | |
| Nilotinib | 2 | (1.7) | 0 | (0.0) | 2 | (4.7) | ||
| Other | 2 | (1.7) | 1 | (1.4) | 1 | (2.3) | ||
| Other | High dose acetaminophen | 1 | (0.9) | 0 | (0.0) | 1 | (2.3) | |
| Temozolomide + imatinib | 1 | (0.9) | 0 | (0.0) | 1 | (2.3) | ||
| Temozolomide + sorafenib | 2 | (1.7) | 2 | (2.7) | 0 | (0.0) | ||
| Total | 117 | (100.0) | 74 | (100.0) | 43 | (100.0) | ||
Immune-based therapy was utilized in 7 (10%) first-line and 13 (30%) second-line regimens, with the most common agent being ipilimumab (N=4 and N=6, respectively). Three patients received PD-1 inhibitors, all in the second-line setting (N=2 nivolumab; N=1 pembrolizumab). Targeted therapy was given to 4 patients (5%) as first-line therapy and to 6 patients (14%) as second-line therapy. Imatinib (N=3 for both first- and second-line) and nilotinib (N=2 second-line) were the most common targeted agents.
Overall Best Response and 24 Week Disease Control Rate
Overall, 14% of patients (10/74; 95% CI: 7–23%) responded to first-line and 23% (10/43; 95% CI: 12–39%) responded to second-line treatments (Table 3). Responses from first-line therapy lasted a median of 12 months (range: 5.3 – 53 months) and from second-line therapy a median of 6 months (range: 3.1 – 64 months). At 24 weeks, disease control rates for first- and second-line therapy were 14%; (10/74; 95% CI: 7–23%) and 7% (3/43; 95% CI: 1–19%), respectively. Best response achieved in the first-line setting was similar for single-agent (2/20, 10%; 95% CI: 1–32%) and combination alkylator therapy (3/38, 8%; 95% CI: 2–21%). By primary site, best response to first-line therapy was 5% for head and neck (1/21; 95% CI: 0–24%), 15% for anorectal (4/27; 95% CI: 4–34%), and 20% for vulvovaginal (5/25; 95% CI: 7–41%) disease. Best response to first-line therapy was 10% (4/39; 95% CI: 3–24%) for patients with liver metastases and 20% (6/30, 95% CI: 8–39%) for those without. Based on cutaneous AJCC M staging, response was 11% (5/47, 95% CI: 4–23%) for M1c, 33% (5/15, 95% CI: 12–62%) for M1a/M1b, and 0% (0/12, 95% CI: 0–26%) for M0. Best response was 16% (3/19, 95% CI: 3–40%) for patients who received adjuvant therapy, 11% (5/44, 95% CI: 4–25%) for patients who did not, and 18% (2/11, 95 CI: 2–52%) for patients who were never free of disease.
Table 3.
Overall Best Response and Disease Control Rate by Type of Therapy and Melanoma Primary Site
| Line | ||||
|---|---|---|---|---|
| Treatment | 1st Line | 2nd Line | ||
| Overall Best Response |
24 wk disease control |
Overall Best Response |
24 wk disease control |
|
| Single Agent Alkylator | 2/20 10% (1–32%) | 2/20 10% (1–32%) | 0/2 0% (0–84%) | 0/2 0% (0–84%) |
| Combination Alkylator | 3/38 8% (2–21%) | 4/38 11% (3–25%) | 4/15 27% (8–55%) | 1/15 7% (0–32%) |
| Other Cytotoxic | 1/3 33% (1–91%) | 2/3 67% (9–99%) | 1/5 20% (1–72%) | 1/5 20% (1–72%) |
| Immune Checkpoint Inhibitors | 1/4 25% (1–81%) | 1/4 25% (1–81%) | 0/10 0% (0–31%) | 0/10 0% (0–31%) |
| Biochemo | 0/3 0% (0–71%) | 0/3 0% (0–71%) | 2/3 67% (9–99%) | 0/3 0% (0–71%) |
| Targeted tx | 1/4 25% (1–81%) | 1/4 25% (1–81%) | 3/6 50% (12–88%) | 1/6 17% (0–64%) |
| Other | 2/2 100% (16–100%) | 0/2 0% (0–84%) | 0/2 0% (0–84%) | 0/2 0% (0–84%) |
| Total | 10/74 14% (7–23%) | 10/74 14% (7–23%) | 10/43 23% (12–39%) | 3/43 7% (1–19%) |
| Line | ||||
|---|---|---|---|---|
| Site | 1st Line | 2nd Line | ||
| Overall Best Response |
24 wk disease control |
Overall Best Response |
24 wk disease control |
|
| Anorectal | 4/27 15% (4–34%) | 5/27 19% (6–38%) | 3/19 16% (3–40%) | 1/19 5% (0–26%) |
| Head and Neck | 1/21 5% (0–24%) | 1/21 5% (0–24%) | 3/8 38% (9–76%) | 1/8 13% (0–53%) |
| Vulvovaginal | 5/25 20% (7–41%) | 4/25 16% (5–36%) | 4/15 27% (8–55%) | 1/15 7% (0–32%) |
| Total | 10/74 14% (7–23%) | 10/74 14% (7–23%) | 10/43 23% (12–39%) | 3/43 7% (1–19%) |
Overall Survival
Median overall survival (OS) from initiation of 1st-line treatment was 10.3 months (95% CI 8.7–13.9 months). OS did not differ by melanoma site (Figure 2), treatment in the first -line setting, sex, race, stage, or age (p=0.10–0.94). However, patients with elevated LDH (HR 1.87, 95% CI: 1.10–3.19, p=0.020) and ECOG 1–2 (HR 1.69, 95% CI: 1.05–2.72, p=0.030) had a higher risk of death. Patients with objective responses by 12 weeks had significantly lower risk of death (HR: 0.12, 95% CI: 0.04–0.41, p<0.001); see Table 4.
Figure 2.
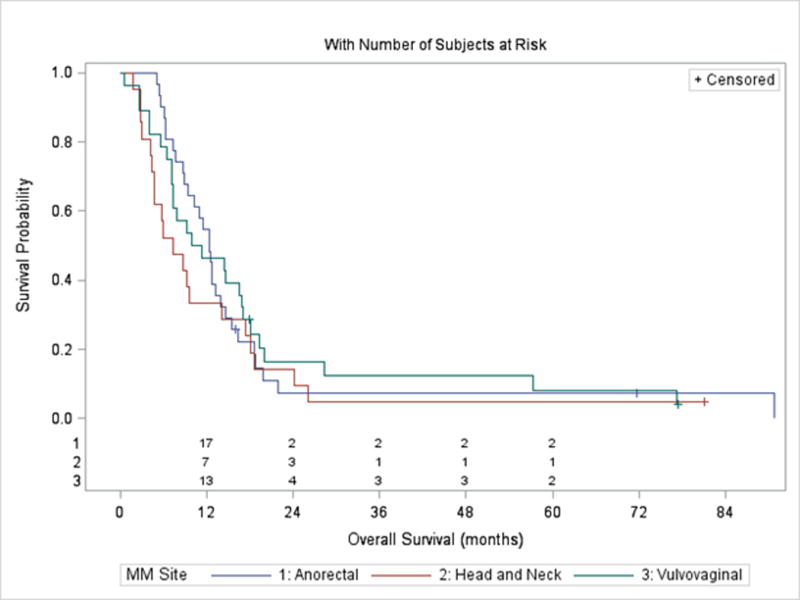
Overall survival from initiation of first systemic treatment by primary site.
Overall OS was 10.3 months (95% CI 8.7–13.9 months).There was no significant difference in median OS for tumors arising from the anorectal, head and neck, and vulvovaginal regions (p=0.57, log-rank test).
Table 4.
Univariate Cox Proportional Hazards Regression for Overall Survival
| HR | [95% | CI] | p-value | ||
|---|---|---|---|---|---|
| Age at Diagnosis | 0.99 | [0.97 – | 1.01] | 0.21 | |
| Site** | Head and Neck | 1.30 | [0.73 – | 2.33] | 0.58 |
| Vulvovaginal | 0.98 | [0.57 – | 1.68] | ||
| Anorectal | REF | ||||
| Gender | Female | 0.73 | [0.44 – | 1.22] | 0.23 |
| Male | REF | ||||
| Race | White | 0.78 | [0.43 – | 1.43] | 0.43 |
| Other | REF | ||||
| Stage | M1c | 1.07 | [0.58 – | 1.99] | 0.10 |
| M1a/M1b | 0.55 | [0.25 – | 1.20] | ||
| M0 | REF | ||||
| LDH Status | Elevated | 1.87 | [1.10 – | 3.19] | 0.020 |
| Normal | REF | ||||
| ECOG PS | 0 | REF | |||
| 1–2 | 1.69 | [1.05 – | 2.72] | 0.030 | |
| Line 1 Subclass (2g) | Combination | REF | |||
| Single Agent | 0.66 | [0.37 – | 1.16] | 0.15 | |
| 12 Week Response* | Responder | 0.12 | [0.04 – | 0.41] | <.001 |
| Non-responder | REF | ||||
Modeled using time dependent covariate
The single patient with a gallbladder primary was excluded from statistical analyses where site was a covariate.
Discussion
To our knowledge, this historical series is the largest to report the effects of cytotoxic therapy in patients with MM. Reporting the initial sites of metastases provides guidance for metastatic surveillance in the post-operative setting. Regardless of primary site, the most common initial sites of metastasis were the lungs, liver, and non-regional lymph nodes. The rate of brain involvement as an initial site of metastasis was 7%, and no primary site was free of risk. Given these data, any surveillance imaging for MM should include the chest, abdomen, and pelvis, and brain imaging could be justified.
Overall, the best response and 24-week disease control rates to systemic therapy were modest in both the first- and second-line settings and are lower than those seen in cutaneous disease. Notably, this historical cohort was weighted toward cytotoxic therapy. Alkylator-based cytotoxic therapy resulted in first-line ORRs of approximately 10%. This ORR is lower than the 16–27% reported in cutaneous melanoma [13, 14] and somewhat lower than the 20% ORR for MM reported in a smaller cohort [8]. This analysis provides an estimate for a historical “non-promising” response rate that can be used to power future clinical trials in mucosal melanoma. Furthermore, this modest activity of cytotoxic therapy in the advanced setting belies the magnitude of survival benefit seen in the adjuvant randomized trial of MM [7], especially given the fact that an adjuvant trial of dacarbazine did not benefit patients in cutaneous melanoma [15]. A second, multicenter randomized trial is needed to confirm the benefit of adjuvant cytotoxic therapy for patients with MM.
We did not detect a significant association between response and single agent versus combination cytotoxic therapy. This analysis could not control for selection bias due to its retrospective design, and the sample size limited our ability to control for other factors in the response analysis. This study nonetheless represents the largest cohort of patients to date receiving these therapies. Given the historically higher toxicity rates of combination cytotoxic regimens [16] and the lack of difference in response rate in this cohort, single agents such as temozolomide or dacarbazine may be preferable for patients with MM who lack other clinical trial options.
In regards to immune checkpoint blockade, the 10 patients receiving ipilimumab in this report were part of a previously reported cohort [17] that reported a 7% RR. Only three patients in our report received anti-PD1 agents, and none derived clinical benefit. Thus, this analysis did not provide additional insight into clinical activity with these standard first-line agents that recently demonstrated ORRs of 23% with nivolumab (95% CI: 15 – 34%) and 37% with nivolumab plus ipilimumab (95% CI: 22 – 55%) [18].
Overall survival from time of initiation of systemic therapy was less than 1 year in this cohort, with no significant differences between MM primary sites. Increased LDH and poor ECOG performance status predicted a higher risk of death, consistent with findings in cutaneous melanomas. While class of cytotoxic therapy was not associated with improved OS, the risk of death was significantly lower in the select group of patients whose tumors have an objective 12 week response to systemic therapy compared to those whose tumors did not respond (HR: 0.12, 95% CI: 0.04–0.41). Further investigation is needed to identify the molecular and/or immunologic mechanisms of mucosal melanoma initiation and progression that will allow us to better select immune-based and other treatments that will benefit these patients.
Acknowledgments
This work was presented in modified form at the American Society of Clinical Oncology 2014 Annual Meeting in Chicago, IL USA. All authors acknowledge the support of the Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748). CB, GB, RH, JAR, and LJF acknowledge grant support from Summer Research Experiences for Medical Students Supervised by Faculty Members (5R25CA020449) under RDC.
Footnotes
Disclosures
The authors have declared no conflicts of interest
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin CC, Wu XC, Jemal A, et al. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103:1000–1007. doi: 10.1002/cncr.20866. [DOI] [PubMed] [Google Scholar]
- 3.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305:2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 5.Carvajal RD, Spencer SA, Lydiatt W. Mucosal melanoma: a clinically and biologically unique disease entity. J Natl Compr Canc Netw. 2012;10:345–356. doi: 10.6004/jnccn.2012.0034. [DOI] [PubMed] [Google Scholar]
- 6.Postow MA, Kuk D, Bogatch K, Carvajal RD. Assessment of overall survival from time of metastastasis in mucosal, uveal, and cutaneous melanoma. ASCO Meeting Abstracts. 2014;32:9074. [Google Scholar]
- 7.Lian B, Si L, Cui C, et al. Phase II Randomized Trial Comparing High-Dose IFN-alpha2b with Temozolomide Plus Cisplatin as Systemic Adjuvant Therapy for Resected Mucosal Melanoma. Clin Cancer Res. 2013;19:4488–4498. doi: 10.1158/1078-0432.CCR-13-0739. [DOI] [PubMed] [Google Scholar]
- 8.Yi JH, Yi SY, Lee HR, et al. Dacarbazine-based chemotherapy as first-line treatment in noncutaneous metastatic melanoma: multicenter, retrospective analysis in Asia. Melanoma Res. 2011;21:223–227. doi: 10.1097/CMR.0b013e3283457743. [DOI] [PubMed] [Google Scholar]
- 9.Kim KB, Sanguino AM, Hodges C, et al. Biochemotherapy in patients with metastatic anorectal mucosal melanoma. Cancer. 2004;100:1478–1483. doi: 10.1002/cncr.20113. [DOI] [PubMed] [Google Scholar]
- 10.Bartell HL, Bedikian AY, Papadopoulos NE, et al. Biochemotherapy in patients with advanced head and neck mucosal melanoma. Head Neck. 2008;30:1592–1598. doi: 10.1002/hed.20910. [DOI] [PubMed] [Google Scholar]
- 11.Harting MS, Kim KB. Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma. Melanoma Res. 2004;14:517–520. doi: 10.1097/00008390-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30:34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol. 1999;17:968–975. doi: 10.1200/JCO.1999.17.3.968. [DOI] [PubMed] [Google Scholar]
- 15.Veronesi U, Adamus J, Aubert C, et al. A randomized trial of adjuvant chemotherapy and immunotherapy in cutaneous melanoma. N Engl J Med. 1982;307:913–916. doi: 10.1056/NEJM198210073071503. [DOI] [PubMed] [Google Scholar]
- 16.Bafaloukos D, Tsoutsos D, Kalofonos H, et al. Temozolomide and cisplatin versus temozolomide in patients with advanced melanoma: a randomized phase II study of the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:950–957. doi: 10.1093/annonc/mdi190. [DOI] [PubMed] [Google Scholar]
- 17.Postow MA, Luke JJ, Bluth MJ, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist. 2013;18:726–732. doi: 10.1634/theoncologist.2012-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J, D'Angelo S, Sosman J, et al. Efficacy and safety of Nivolumab (NIVO) monotherapy in the treatment of advanced mucosal melanoma (MEL) Pigment Cell & Melanoma Research. 2015;28:789. [Google Scholar]


