Abstract
Objectives
Test the hypothesis that inhibiting mammalian target of rapamycin (mTOR) and Insulin Growth Factor-1 Receptor would be efficacious in metastatic uveal melanoma.
Methods
Phase 2 trial of everolimus 10mg daily plus pasireotide long-acting release 60mg every 28 days enrolling patients with progressive, metastatic uveal melanoma to treatment until progression by RECIST 1.1 or unacceptable toxicity. The primary endpoint was clinical benefit rate (CBR), defined as any objective response or RECIST 1.1 stable disease at 16 weeks. A subset of patients underwent baseline Indium-111 octreotide scans.
Results
14 patients were enrolled, of which 13 were evaluable for the primary endpoint, before the study was terminated due to poor accrual. Three of 13 (26%) patients obtained clinical benefit. Seven of 13 (54%) had stable disease lasting a median of 8 weeks (range: 8–16 weeks). Grade 3 adverse events deemed at least possibly related to study drugs were hyperglycemia (n=7), oral mucositis (n=2), diarrhea (n=1), hypophosphatemia (n=1), and anemia (n=1). Seven of 14 patients (50%) required at least 1 dose reduction due to toxicity. Seven of 8 patients (88%) with baseline In111 octreotide scans had at least 1 avid lesion, with significant intrapatient heterogeneity. There was a trend toward an association between octreotide avidity and cytostatic response to therapy (p=0.078).
Conclusions
The combination of everolimus and pasireotide has limited clinical benefit in this small metastatic uveal melanoma cohort. Dose reductions for side effects were common. Further investigation into the relationship between somatostatin receptor expression and cytostatic activity of somatostatin analogues is warranted.
Keywords: uveal melanoma, everolimus, mTOR, somatostatin, pasireotide, insulin growth factor, octreotide
Background
Uveal melanoma is the most common primary intraocular malignancy in adults, with an incidence of 4–5 cases per million [1]. Approximately half of all patients with uveal melanoma will eventually develop metastatic disease, and their prognosis remains poor [2]. Over the past several years, studies have identified activating mutations in the G alpha protein subunits GNAQ and GNA11 in the vast majority of uveal melanomas that lead to downstream activation of multiple signaling pathways, including mitogen-activated protein kinase (MAPK), Protein Kinase C (PKC), and Phosphoinositol-3-Kinase (PI3K)/Akt/mammalian target of Rapamycin (mTOR) [3–6]. Recently, a randomized Phase 2 trial of the MEK inhibitor selumetinib demonstrated a progression-free survival (PFS) benefit versus investigator’s choice chemotherapy (median 16 vs 8 weeks) [7]. This has provided proof of concept that targeted inhibition of growth signals in uveal melanoma can lead to clinical benefit. Recently, a Phase 3 trial of dacarbazine versus dacarbazine plus selumetinib failed to demonstrate an improvement in progression free survival, suggesting combined inhibition of multiple signaling pathways may be required for sustained clinical benefit.
PI3K/Akt/mTOR signaling is dysregulated in a variety of human cancers [8]. In uveal melanoma, 60% of metastatic tumors display loss of PTEN, a tumor suppressor that inhibits this pathway [9]. As a result, mTOR represents an attractive therapeutic target in uveal melanomas. Clinical resistance to mTOR monotherapy is a frequent occurrence, however, and combination therapeutic strategies may be required for sustained clinical benefit. One potential mechanism of resistance to mTOR inhibition is rebound activation of insulin-like growth factor-1 receptor (IGF1R) pathway signaling [10]. In uveal melanoma, tumor IGF1R expression has been associated with disease progression and in vitro inhibition of IGF1R causes uveal melanoma tumor regression [11, 12].
IGF1 ligand levels in plasma are increased by somatostatin signaling, and in one study of 25 uveal melanoma specimens, 100% expressed somatostatin receptor (SSTR)-2A, 56% expressed SSTR-5, and 28% expressed SSTR-3 [13]. This suggests that nuclear imaging with an octreotide analog such as Indium-111 octreotide (Octreoscan) that binds to SSTR-2A may be used to select for patients with advanced uveal melanoma that may be more likely to respond to IGF1R pathway inhibition. A recent report by Valsecchi and colleagues [14] demonstrated In111 octreotide avidity in 14/30 patients (44%) with advanced uveal melanoma. In their cohort, 7 patients with positive In111 octreotide scans received Sandostatin long-acting release (LAR) outside of a formal clinical trial, and two patients had clinically stable disease for over 5 months [14].
Combination IGF1R and mTOR inhibition therefore may provide clinical benefit in uveal melanoma. To investigate this, we launched a single-arm Phase 2 trial of combined mTOR and IGF1R inhibition with everolimus and pasireotide LAR in patients with metastatic uveal melanoma. Correlative analysis was performed by measuring plasma levels of IGF1 and baseline Indium-111 (In111) octreotide scans. We hypothesized that plasma IGF1 levels would decrease while receiving pasireotide LAR, consistent with IGF1R pathway inhibition, and patients with positive In111 octreotide uptake in their tumors would derive more benefit from the study combination than those whose tumors did not take up In111 octreotide.
Methods
Trial Design
This is an open-label, single-arm Phase II trial in which patients with metastatic uveal melanoma received everolimus (RAD001) 10mg orally daily plus pasireotide LAR (SOM230) 60mg intramuscularly (IM) once every 28 days until progression by RECIST 1.1 or unacceptable toxicity. Up to two dose reductions for both everolimus and pasireotide LAR were allowed to accommodate for toxicities. This protocol (NCT01252251) was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Key inclusion criteria were age >18 years, histologically confirmed metastatic UM with measurable disease per RECIST 1.1, progression on prior therapy in the opinion of the treating physician, ECOG performance status (PS) 0–1, and adequate end-organ function. Key exclusion criteria included brain metastases with stability <2 months, prior therapy with an mTOR inhibitor, or uncontrolled hyperglycemia or hypertriglyceridemia. Patients were evaluated via cross-sectional imaging at baseline and then every 8 weeks, corresponding to the end of even numbered cycles. Adverse events were coded using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
The primary endpoint was clinical benefit rate (CBR), defined as RECIST 1.1 stable disease at 16 weeks or any objective response. Secondary endpoints included PFS and overall survival (OS). Patients who did not complete 1 cycle (28 days) due to toxicity were considered replaceable for purposes of the primary endpoint.
Imaging and Plasma Correlates
Optional In111 octreotide scans were performed prior to starting therapy using planar imaging done at 4 hrs and 24 hours post injection of radiotracer. SPECT or SPECT/CT of the abdomen was performed at 4 hours post injection, and SPECT or SPECT/CT of the chest at 24 hours post injection. All scans were retrospectively reviewed by a board certified nuclear medicine physician on a Hermes workstation (Hermes Medical Solutions, Stockholm, Sweden). A 1cm 3D spherical region of interest was placed over all malignant lesions noted by the study radiologist, and 3Dmax and mean counts were recorded. Max and mean counts from normal liver and aortic arch (for blood pool) were recorded to serve as reference regions. Mean background counts were used in determining tumor to liver and tumor to blood pool ratios. Ratios of uptake in lesions versus background were used to derive semiquantitative assessment of uptake intensity, and any lesion with a ratio >2 above background aortic arch uptake at either 4 or 24 hours was considered avid.
Serum insulin-like growth factor-1 (IGF1) levels were assayed at baseline and after every 4 weeks using a commercial liquid chromatography-mass spectrometry assay (Quest Diagnostics, San Juan Capistrano, CA) to assess changes in the IGF1R signaling axis.
Statistical Methods
A Simon two-stage design was utilized to evaluate the CBR. In order to evaluate a promising CBR of 30% versus non-promising CBR of 10% with 90% power at an alpha of 0.10, ≥5 of 25 total patients would have to achieve clinical benefit. The trial was designed to terminate for futility if ≤1 of the first 16 patients achieved clinical benefit.
To explore the association between study drug response and In111 octreotide avidity, each RECIST 1.1 target lesion was assessed from baseline to first follow up CT scan and classified as an In111 octreotide avid or non-avid lesion. Since multiple lesions were measured within each patient, a generalized estimating equation model was utilized to assess association between In111 octreotide avidity of the lesion and percentage changes in size by RECIST. P values <0.05 were considered significant.
Results
Demographics
Between 1/1/2011 and 4/31/2014, 21 patients were consented, of which 14 enrolled on study. The clinical characteristics of these 14 patients are listed in Table 1. Median age was 61 (range: 41–83). Median ECOG PS was 0 (range: 0–1). The majority of patients had M1b or M1c disease (64 and 14%, respectively) by AJCC 7th edition staging guidelines for uveal melanoma [15]. Median number of prior systemic therapy was 2 (range: 1–5), with 9/14 (64%) having progressed on a prior MEK inhibitor. Six patients also received at least 1 prior locoregional therapy. GNAQ/11 Exon 5 mutational status was assessed in 12/14 patients. 10 of these 12 patients had Q209 mutations (GNAQ n=5, GNA11 n=5). Of the two Exon 5 wild-type samples, 1 had a GNA11 Exon 4 R183Q mutation and 1 was not tested further.
Table 1.
Patient Demographics
| Patient # | Sex | Age | Uveal Stage | Cutaneous Stage | LDH* | ECOG | # Prior Systemic Therapies | Prior MEK Inhibitor? | # Prior Local Therapies | GNAQ/11 | Codon |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 61 | M1b | M1c | High | 0 | 3 | Yes | 1 | GNAQ | Q209L |
| 2 | M | 66 | M1b | M1c | High | 0 | 2 | Yes | 1 | GNAQ | Q209P |
| 3 | M | 55 | M1b | M1c | Normal | 0 | 2 | Yes | 0 | GNAQ | Q209L |
| 4 | F | 63 | M1b | M1c | Normal | 0 | 2 | Yes | 0 | GNA11 | Q209L |
| 5 | M | 61 | M1b | M1c | Normal | 1 | 2 | Yes | 0 | GNA11 | Q209L |
| 6 | F | 52 | M1a | M1b | Normal | 0 | 1 | Yes | 0 | GNAQ | Q209L |
| 7 | F | 72 | M1c | M1c | High | 0 | 2 | Yes | 0 | not done | not done |
| 8 | F | 42 | M1b | M1c | High | 0 | 1 | No | 1 | unknown | Ex5 wt |
| 9 | F | 72 | M1b | M1c | High | 0 | 1 | Yes | 0 | GNA11 | Q209L |
| 10 | F | 74 | M1a | M1c | High | 0 | 5 | Yes | 0 | GNA11 | R183Q |
| 11 | F | 60 | M1a | M1c | High | 1 | 4 | No | 5 | GNA11 | Q209L |
| 12 | M | 56 | M1b | M1c | High | 0 | 3 | No | 0 | GNA11 | Q209L |
| 13 | M | 83 | M1b | M1c | High | 0 | 1 | No | 1 | not done | not done |
| 14 | M | 58 | M1c | M1c | High | 0 | 3 | No | 3 | GNAQ | Q209R |
High refers to a value above the upper limit of normal
Efficacy
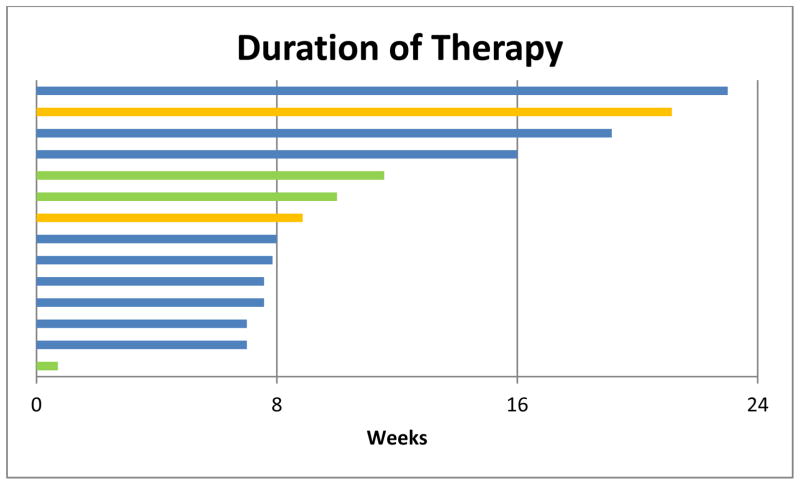
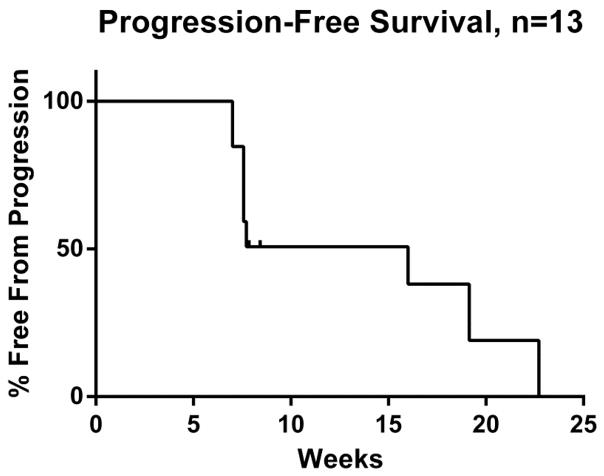
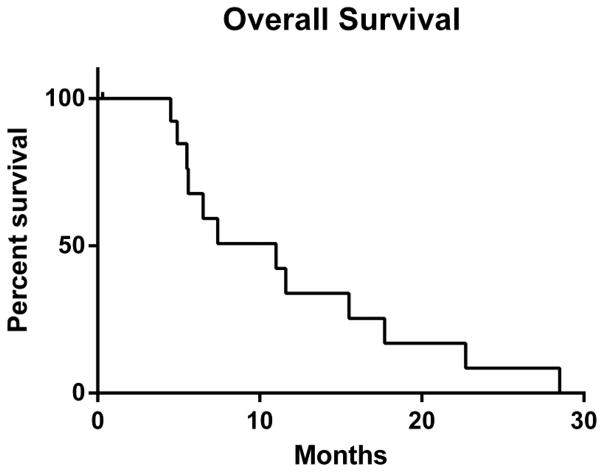
The study was terminated early due to poor accrual. Thirteen of 14 patients were evaluable for the primary endpoint; one patient withdrew consent after less than one week on study. See Table 2 for efficacy for each patient on study. Three of 13 patients (23%) had stable disease for at least 16 weeks and were considered to have derived clinical benefit. Overall, SD was the best objective outcome in 7 of 13 patients. Median duration of SD in these patients was 8 weeks (range: 8–16 weeks). Six of 13 patients had PD on first assessment. Two patients had disease regression (−10% and −28%), both of which had tumors that were wild-type for GNAQ/11 Exon 5 mutations. Median time on treatment was 8 weeks (range: 1–23 weeks); see Figure 1. Reasons for stopping therapy were disease progression (n=9), toxicity (n=3), or a switch to attempt a newly available alternate therapy (n=2). Median PFS from first date of treatment for the 13 evaluable patients was 16 weeks (range: 7–23 weeks); see Figure 2. Median OS from first day of study treatment was 11 months (range: 4.5–28.5 months); see Figure 3.
Table 2.
Efficacy outcomes, IGF1 suppression, and In111 octreotide results
| Patient # | RECIST Best Response | Best % Change in Tumor Burden | Duration of SD (weeks) | Time on Study (weeks) | Clinical Benefit? | IGF1 Suppression after 1 Cycle | Octreotide Avidity (+/total lesions) |
|---|---|---|---|---|---|---|---|
| 1 | POD | +20.3 | N/A | 8 | No | 57% | Yes (1/3) |
| 2 | SD | +3 | 16.0 | 21 | Yes | 56% | Yes (3/4) |
| 3 | POD | +6.5 | N/A | 7 | No | 23% | Yes (1/4) |
| 4 | N/A | N/A | N/A | 0.7 | N/A | N/A | N/A |
| 5 | SD | +14 | 8.4 | 12 | No | 7% | Yes (3/6) |
| 6 | SD | +3 | 7.0 | 10 | No | 39% | Yes (2/2) |
| 7 | SD | +4 | 15.0 | 19 | Yes | 51% | Yes (3/6) |
| 8 | SD | −28 | 7.9 | 16 | No | 42% | N/A |
| 9 | POD | +53 | N/A | 8 | No | 63% | N/A |
| 10 | SD | −10 | 15.4 | 23 | Yes | 74% | N/A |
| 11 | POD | +20 | N/A | 8 | No | 26% | N/A |
| 12 | SD | +16 | 7.9 | 9 | No | 54% | N/A |
| 13 | POD | +35 | N/A | 8 | No | 75% | Yes (1/1) |
| 14 | POD | +35 | N/A | 7 | No | 69% | No (0/2) |
Figure 1.
Swim plot of treatment duration on study and reasons for discontinuation. Three patients obtained >16 weeks of clinical benefit. Patients came off for either POD (blue; n=9), toxicity (green; n=3), or pursuing immune-based therapy (orange; n=2).
Figure 2.
Kaplan-Meier curve of progression-free survival. Median PFS was 16 weeks.
Figure 3.
Kaplan-Meier curve of overall survival. Median OS was 11 months (range: 4.5 – 28.5 months).
Toxicity
Table 3 depicts all adverse events (AEs) occurring in >1 patient and Grade 3 AEs occurring in ≥1 patient deemed at least possibly related to study drugs. All 14 patients experienced at least 1 possibly related AE. The most frequently identified AEs were metabolic (hyperglycemia, hypertriglyceridemia, hypercholesterolemia), gastrointestinal (diarrhea, oral mucositis), or hematologic (leukopenia, thrombocytopenia, neutropenia) in nature. The Grade 3 AEs deemed at least possibly related were hyperglycemia (n=7), oral mucositis (n=2), diarrhea (n=1), hypophosphatemia (n=1), and anemia (n=1). There were no Grade 4 or 5 AEs.
Table 3.
Select CTCAE v4.0 Adverse Events at least Possibly Related to Study Drugs
| Adverse Event | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Hyperglycemia | 1 (7%) | 4 (29%) | 7 (50%) |
| Hypertriglyceridemia | 8 (57%) | 2 (14%) | 0 |
| Diarrhea | 8 (57%) | 0 | 1 (7%) |
| Leukopenia | 5 (36%) | 4 (29%) | 0 |
| Hypercholesterolemia | 4 (29%) | 4 (29%) | 0 |
| Mucositis | 4 (29%) | 2 (14%) | 2 (14%) |
| Thrombocytopenia | 7 (50%) | 0 | 0 |
| Neutropenia | 0 | 5 (36%) | 0 |
| Elevated CPK | 4 (29%) | 0 | 0 |
| Hypophosphatemia | 0 | 2 (14%) | 1 (7%) |
| Nausea | 3 (21%) | 0 | 0 |
| Rash | 2 (14%) | 1 (7%) | 0 |
| Dyspnea | 1 (7%) | 1 (7%) | 0 |
| Fatigue | 2 (14%) | 0 | 0 |
| Anemia | 0 | 0 | 1 (7%) |
All AEs occurring in >1 patient regardless of severity or any Grade 3 or higher AEs at least possibly related to study drugs are depicted. There were no Grade 4 or 5 toxicities related to study drugs.
Seven of 14 patients (50%) had at least 1 dose reduction of everolimus. The reasons for dose reduction were mucositis (n=6) and anemia (n=1). Three patients discontinued study treatment due to toxicity: 2 for Grade 2 mucositis and 1 for Grade 3 diarrhea.
Indium111 Octreotide Scans, Plasma IGF1R Levels, and Outcomes
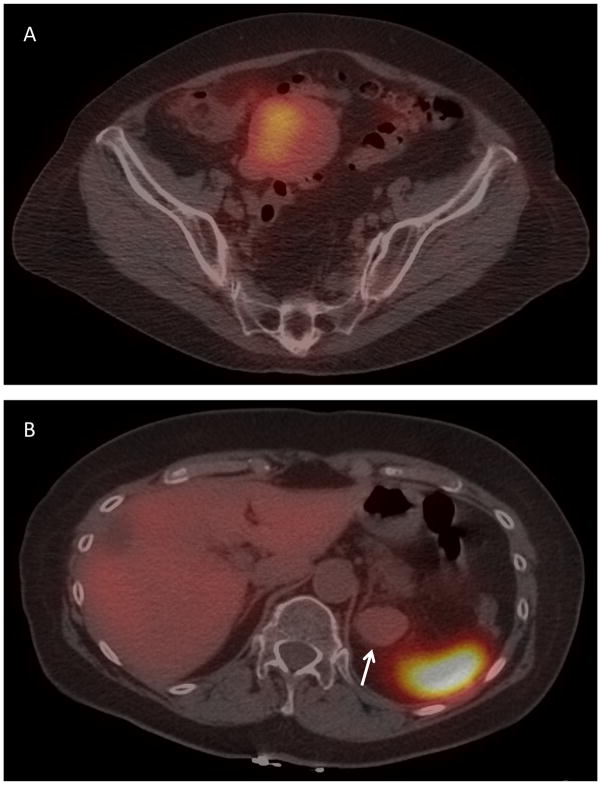
8 patients who were evaluable for response underwent baseline Indium-111 octreotide scans, of which 7 of 8 (88%) had positive uptake in ≥1 metastatic lesion. There was heterogeneous uptake in most patients (Table 2 and Figure 4). Five of 7 patients with >1 assessable lesion had both In111 octreotide avid and non-avid tumors. Overall, 14 of 28 lesions (50%) were In111 octreotide avid. Among the 26 lesions that had been labeled prospectively as RECIST 1.1 target lesions, 12 were avid. There was a trend toward In111 avidity and lower percent growth in lesion size on therapy that did not meet statistical significance (p=0.078).
Figure 4.
Heterogeneous In111 octreotide pre-treatment uptake in a patient with metastatic uveal melanoma. (A) Pelvic metastasis with In111 octreotide avidity. (B) Left perinephric metastasis (arrow) without In111 octreotide avidity (with adjacent normal avid spleen visible).
In the 13 evaluable patients, plasma IGF1 levels after 1 cycle of therapy were decreased a median of 54% compared to baseline (range: 7–75%), which was statistically significant (p<0.001, paired t test). This reduction in IGF1 levels was sustained for patients who remained on therapy (data not shown). There was no association between IGF1 plasma levels and clinical benefit.
Discussion
Metastatic uveal melanoma remains a disease with poor prognosis, with most patients surviving 6–12 months following diagnosis of metastasis [16, 17]. Multiple ongoing clinical trials are investigating the clinical benefit of inhibiting MEK, PKC, and/or PI3K, and there is a critical need to identify relevant therapeutic targets in this disease [18].
In this highly-pretreated cohort of patients with metastatic uveal melanoma, there was preliminary evidence of clinical benefit with combined IGF-1R and mTOR inhibition. Although the trial did not complete accrual, the fact that 3 patients obtained >16 weeks of stable disease suggests certain patients with advanced uveal melanoma may benefit from this therapy. Interestingly, all 3 of these patients had previously progressed on MEK inhibitors (selumetinib, n=2; trametinib, n=1). Recent work has identified that Akt upregulation is common in MEK-resistant cell lines and showed HRAS signaling is an important upstream mediator [19]. The possibility that mTOR and/or IGF-1 signaling plays some additional role in progression to MEK inhibitors requires further investigation. There were no on-treatment biopsies to assess the pharmacodynamic effect of everolimus in this study. The decrease in serum IGF-1 levels, however, suggests pasireotide LAR inhibited signaling of the IGF-1 axis to varying extents.
Of the 8 evaluable patients with an In111-octreotide scan, 7 (88%) had at least 1 octreotide-avid metastasis. This rate is somewhat higher than the 44% rate described by Valsecchi et al despite using the same cutoff for positivity of two-fold increase above background [14]. Our study is the first, to our knowledge, to describe intrapatient heterogeneity in In111 octreotide avidity between multiple tumors and suggests there is intrapatient heterogeneity in tumor expression of SSTR-2A. In this small sample size, there was a trend between In111 octreotide avidity and stability of disease with combined anti-IGF1R plus mTOR therapy that did not reach statistical significance. This suggests that, while the routine use of In111-octreotide scans in this disease cannot be recommended to document extent of metastatic disease, there may be a biologically relevant relationship between In111 octreotide avidity and cytostatic effect of somatostatin analogues in uveal melanoma. This should be investigated in a larger cohort of patients, potentially utilizing more sensitive nuclear imaging modalities such as Gallium-68 octreotate positron emission tomography [20].
As our understanding of uveal melanoma biology increases and more clinical trials utilize targeted inhibitors of growth signals, it will become increasingly important to identify rational strategies to combine antineoplastic agents. This study is the first to our knowledge to combine targeted inhibition in uveal melanoma. Combination therapy with mTOR and IGF1-R inhibitors led to a relatively high rate of AEs, most commonly metabolic, GI, and hematologic in nature. Dose reductions, largely for mucositis, may have limited the long-term tolerability of this combination and slowed accrual of this trial. Nonetheless, given the presence of prolonged stable disease in select patients refractory to multiple therapies, future trials investigating the role of mTOR and/or IGF1-R inhibition in uveal melanoma are warranted.
Acknowledgments
Funding: Novartis
Core grant P30 CA008748 to ANS, MAP, MKC, JW, PBC, and KSP.
Footnotes
Conflict of Interest: GKS receives compensation from Novartis for advisory board work and RDC receives compensation for consulting. The remainder of authors have no declarations.
References
- 1.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 3.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2013 doi: 10.1038/onc.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalili JS, Yu X, Wang J, et al. Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ- and GNA11-dependent manner. Clin Cancer Res. 2012;18:4345–4355. doi: 10.1158/1078-0432.CCR-11-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. Jama. 2014;311:2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Rahman MH, Yang Y, Zhou XP, et al. High frequency of submicroscopic hemizygous deletion is a major mechanism of loss of expression of PTEN in uveal melanoma. J Clin Oncol. 2006;24:288–295. doi: 10.1200/JCO.2005.02.2418. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girnita A, All-Ericsson C, Economou MA, et al. The insulin-like growth factor-I receptor inhibitor picropodophyllin causes tumor regression and attenuates mechanisms involved in invasion of uveal melanoma cells. Clin Cancer Res. 2006;12:1383–1391. doi: 10.1158/1078-0432.CCR-05-1106. [DOI] [PubMed] [Google Scholar]
- 12.All-Ericsson C, Girnita L, Seregard S, et al. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43:1–8. [PubMed] [Google Scholar]
- 13.Ardjomand N, Ardjomand N, Schaffler G, et al. Expression of somatostatin receptors in uveal melanomas. Invest Ophthalmol Vis Sci. 2003;44:980–987. doi: 10.1167/iovs.02-0481. [DOI] [PubMed] [Google Scholar]
- 14.Valsecchi ME, Coronel M, Intenzo CM, et al. Somatostatin receptor scintigraphy in patients with metastatic uveal melanoma. Melanoma Res. 2013;23:33–39. doi: 10.1097/CMR.0b013e32835b70e9. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Cerbone L, Van Ginderdeuren R, Van den Oord J, et al. Clinical presentation, pathological features and natural course of metastatic uveal melanoma, an orphan and commonly fatal disease. Oncology. 2014;86:185–189. doi: 10.1159/000358729. [DOI] [PubMed] [Google Scholar]
- 17.Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. Journal of Clinical Oncology. 2005;23:8076–8080. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 18.Shoushtari AN, Carvajal RD. GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res. 2014;24:525–534. doi: 10.1097/CMR.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini G, Khanin R, Carvajal RD, Schwartz GK. Overexpression of DDX43 mediates MEK inhibitor resistance through RAS Upregulation in uveal melanoma cells. Mol Cancer Ther. 2014;13:2073–2080. doi: 10.1158/1535-7163.MCT-14-0095. [DOI] [PubMed] [Google Scholar]
- 20.Hofman MS, Kong G, Neels OC, et al. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]