Abstract
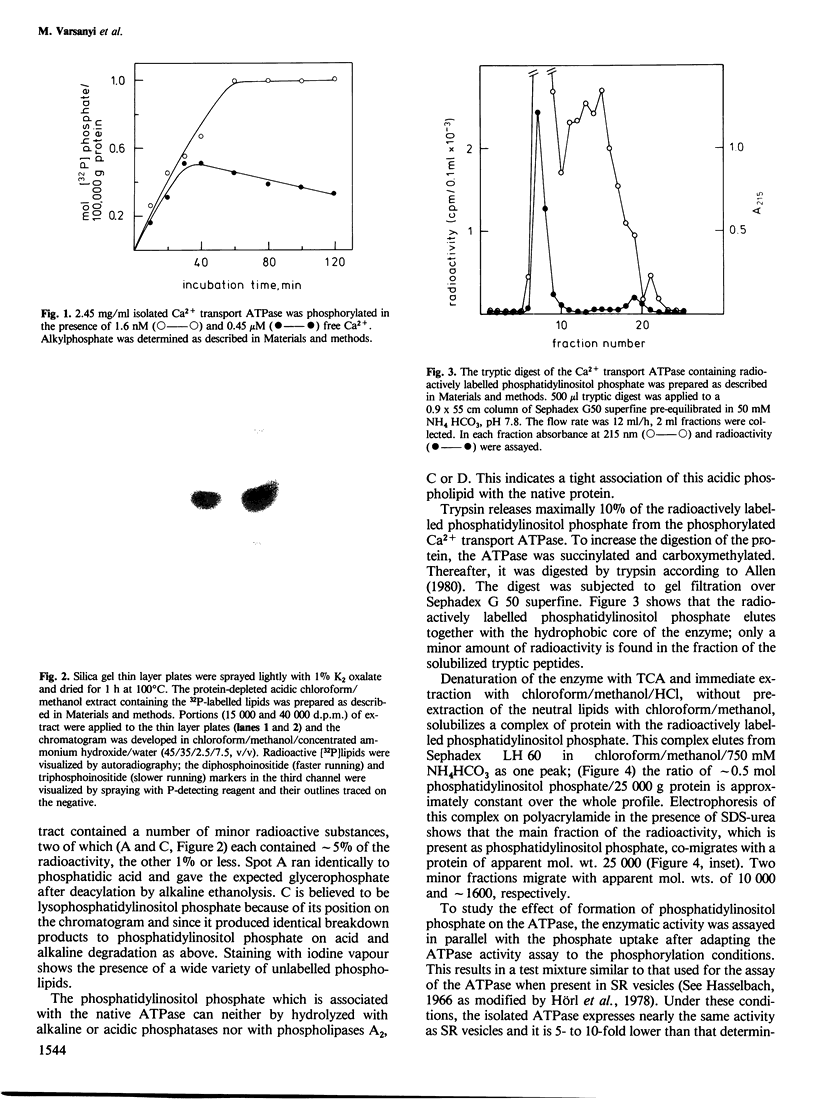
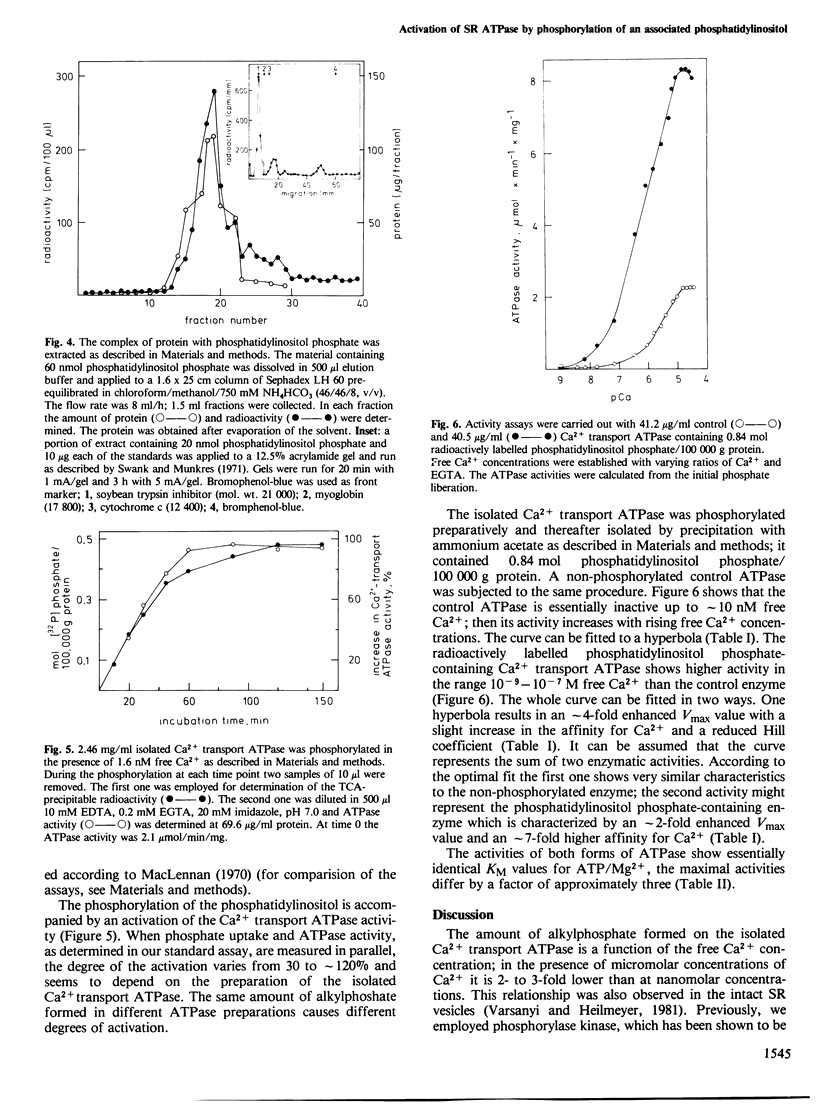
Approximately 1 mol phosphatidylinositol phosphate is formed per mol isolated Ca2+ transport ATPase when the enzyme is incubated with ATP/Mg2+. The phosphorylation of this enzyme-associated phosphatidylinositol represents the alkylphosphate formation described earlier. The phosphatidylinositol phosphate has been found in the hydrophobic core of the enzyme. A complex of phosphatidylinositol phosphate with protein can be extracted with acidic chloroform/methanol. The protein behaves like proteolipid during chromatography on Sephadex LH 60 and binds the radioactively labelled phosphatidylinositol phosphate. The phosphorylation of approximately 1 mol phosphatidylinositol per 100,000 g protein correlates with an enhancement of the Ca2+ transport ATPase activity which is due to an approximately 7-fold enhanced affinity for Ca2+ and an approximately 2-fold enhanced maximal turnover.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. The primary structure of the calcium-transporting adenosine triphosphatase of rabbit skeletal sarcoplasmic reticulum. Soluble tryptic peptides from the succinylated carboxymethylated protein. Biochem J. 1980 Jun 1;187(3):545–563. doi: 10.1042/bj1870545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- DAWSON R. M., DITTMER J. C. Evidence for the structure of brain triphosphoinositide from hydrolytic degradation studies. Biochem J. 1961 Dec;81:540–545. doi: 10.1042/bj0810540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., DAWSON R. M. The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem J. 1961 Dec;81:535–540. doi: 10.1042/bj0810535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meis L., Hasselbach W. Acetyl phosphate as substrate for Ca 2+ uptake in skeletal muscle microsomes. Inhibition by alkali ions. J Biol Chem. 1971 Aug 10;246(15):4759–4763. [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M., Kuriki Y., Biltonen R., Racker E. Calorimetric studies of ligand-induced modulation of calcium adenosine 5'-triphosphatase from sarcoplasmic reticulum. Biochemistry. 1980 Nov 25;19(24):5564–5568. doi: 10.1021/bi00565a016. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W. Structural and enzymatic properties of the calcium transporting membranes of the sarcoplasmic reticulum. Ann N Y Acad Sci. 1966 Jul 14;137(2):1041–1048. doi: 10.1111/j.1749-6632.1966.tb50216.x. [DOI] [PubMed] [Google Scholar]
- Hörl W. H., Heilmeyer L. M., Jr Evidence for the participation of a Ca2+-dependent protein kinase and protein phosphatase in the regulation of the Ca2+ transport ATPase of the sarcoplasmic reticulum. 2. Effect of phosphorylase kinase and phosphorylase phosphatase. Biochemistry. 1978 Mar 7;17(5):766–772. doi: 10.1021/bi00598a002. [DOI] [PubMed] [Google Scholar]
- Hörl W. H., Jennissen H. P., Heilmeyer L. M., Jr Evidence for the participation of a Ca2+-dependent protein kinase and a protein phosphatase in the regulation of the Ca2+ transport ATPase of the sarcoplasmic reticulum. 1. Effect of inhibitors of the Ca2+-dependent protein kinase and protein phosphatase. Biochemistry. 1978 Mar 7;17(5):759–766. doi: 10.1021/bi00598a001. [DOI] [PubMed] [Google Scholar]
- Jahnke U., Heilmeyer L. M., Jr Comparison of the Mg2+ and Ca2+ binding properties of troponin complexes P1-TI2C and TI2C. Eur J Biochem. 1980 Oct;111(2):325–332. doi: 10.1111/j.1432-1033.1980.tb04945.x. [DOI] [PubMed] [Google Scholar]
- Kalbitzer H. R., Stehlik D. On the analysis of competitive binding of various ligands to cooperative and independent binding sites of macromolecules. Z Naturforsch C. 1979 Sep-Oct;34(9-10):757–769. doi: 10.1515/znc-1979-9-1018. [DOI] [PubMed] [Google Scholar]
- Kondratiuk T. P., Kurskii M. D., Fedorov A. N., Osipenko A. A., Meshkova L. I., Litvinenko E. A. Kharakteristika substratov endogennogo fosforilirovaniia fragmentov sarkoplazmaticheskogo retikuluma bystrykh skeletnykh myshts krolika. Biokhimiia. 1982 Jun;47(6):950–956. [PubMed] [Google Scholar]
- Kurskii M. D., Kondratiuk T. P., Osipenko A. A., Fedorov A. N., Grigor'eva V. A. Endogennoe fosforilirovanie fragmentov sarkoplazmaticheskogo retikuluma bystrykh skeletnykh myshts krolika. Biokhimiia. 1982 Jan;47(1):34–42. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Makinose M. The phosphorylation of the membranal protein of the sarcoplasmic vesicles during active calcium transport. Eur J Biochem. 1969 Aug;10(1):74–82. [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Characterization of sarcoplasmic reticulum from skeletal muscle. Biochim Biophys Acta. 1971 Aug 13;241(2):356–378. doi: 10.1016/0005-2736(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Niggli V., Penniston J. T., Carafoli E. Purification of the (Ca2+-Mg2+)-ATPase from human erythrocyte membranes using a calmodulin affinity column. J Biol Chem. 1979 Oct 25;254(20):9955–9958. [PubMed] [Google Scholar]
- Racker E., Eytan E. A coupling factor from sarcoplasmic reticulum required for the translocation of Ca2+ ions in a reconstituted Ca2+ATPase pump. J Biol Chem. 1975 Sep 25;250(18):7533–7534. [PubMed] [Google Scholar]
- Redman C. M. Proteolipid involvement in human erythrocyte membrane function. Biochim Biophys Acta. 1972 Sep 1;282(1):123–134. doi: 10.1016/0005-2736(72)90316-1. [DOI] [PubMed] [Google Scholar]
- Richards D. E., Irvine R. F., Dawson R. M. Hydrolysis of membrane phospholipids by phospholipases of rat liver lysosomes. Biochem J. 1979 Aug 15;182(2):599–606. doi: 10.1042/bj1820599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Swoboda G., Fritzsche J., Hasselbach W. Effects of phospholipase A2 and albumin on the calcium-dependent ATPase and the lipid composition of sarcoplasmic membranes. Eur J Biochem. 1979 Mar 15;95(1):77–88. doi: 10.1111/j.1432-1033.1979.tb12941.x. [DOI] [PubMed] [Google Scholar]
- Varsanyi M., Heilmeyer L. M., Jr Phosphorylation of the 100 000 Mr Ca2+-transport ATPase by Ca2+ or cyclic AMP-dependent and -independent protein kinases. FEBS Lett. 1981 Aug 31;131(2):223–228. doi: 10.1016/0014-5793(81)80372-9. [DOI] [PubMed] [Google Scholar]
- Varsànyi M., Gröschel-Stewart U., Heilmeyer M. G., Jr Characterization of a Ca2+ -dependent protein kinase in skeletal muscle membranes of I-strain and wild-type mice. Eur J Biochem. 1978 Jun 15;87(2):331–340. doi: 10.1111/j.1432-1033.1978.tb12382.x. [DOI] [PubMed] [Google Scholar]
- Varsányi M., Heilmeyer L. M., Jr Ca2+ regulation of sarcoplasmic reticular protein phosphatase activity. Biochemistry. 1979 Oct 30;18(22):4869–4875. doi: 10.1021/bi00589a015. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Lewis D., Nakamoto R., Kurzmack M., Fronticelli C., Inesi G. Modulation of calcium binding in sarcoplasmic reticulum adenosinetriphosphatase. Biochemistry. 1981 Nov 10;20(23):6617–6625. doi: 10.1021/bi00526a015. [DOI] [PubMed] [Google Scholar]
- de Meis L., Tume R. K. A new mechanism by which an H+ concentration gradient drives the synthesis of adenosine triphosphate, pH jump, and adenosine triphosphate synthesis by the Ca2+-dependnet adenosine triphosphatase of sarcoplasmic reticulum. Biochemistry. 1977 Oct 4;16(20):4455–4463. doi: 10.1021/bi00639a020. [DOI] [PubMed] [Google Scholar]



