Abstract
A recent study suggested that insulin resistance may play a central role in the pathogenesis of Alzheimer's disease (AD). In this regard, it is of note that upregulation of plasma adiponectin (APN), a benign adipokine that sensitizes the insulin receptor signaling pathway and suppresses inflammation, has recently been associated with the severities of amyloid deposits and cognitive deficits in the elderly, suggesting that APN may enhance the risk of AD. These results are unanticipated because AD has been linked to type II diabetes and other metabolic disorders in which hypoadiponectinemia has been firmly established, and because APN ameliorated neuropathological features in a mouse model of neurodegeneration. Therefore, the objective of this study is to discuss the possible mechanisms underlying the biological actions of APN in the context of AD. Given that insulin receptor signaling is required for normal function of the nervous system, we predict that APN may be upregulated to compensate for compromised activity of the insulin receptor signaling pathway. However, increased APN might be sequestered by tau in the brain, leading to neurotoxic protein aggregation in AD. Alternatively, misfolding of APN may result in downregulation of the insulin/APN signal transduction network, leading to decreased neuroprotective and neurotrophic activities. Thus, it is possible that both ‘gain of function’ and ‘loss of function’ of APN may underlie synaptic dysfunction and neuronal cell death in AD. Such a unique biological mechanism underlying APN function in AD may require a novel therapeutic strategy that is distinct from previous treatment for metabolic disorders.
Introduction
A growing body of evidence suggests that metabolic dysfunction may play a major role in the pathogenesis of Alzheimer's disease (AD) and related neurodegenerative disorders.1 In support of this idea, various lifestyle‐related disorders, such as type II diabetes (T2DM), dyslipidemia, and obesity, have been epidemiologically linked to AD.2 Furthermore, AD and these metabolic disorders are commonly associated with a number of pathological features, including impairment of insulin signaling, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, oxidative stress, protein aggregation, and inflammation (Fig. 1).3
Figure 1.
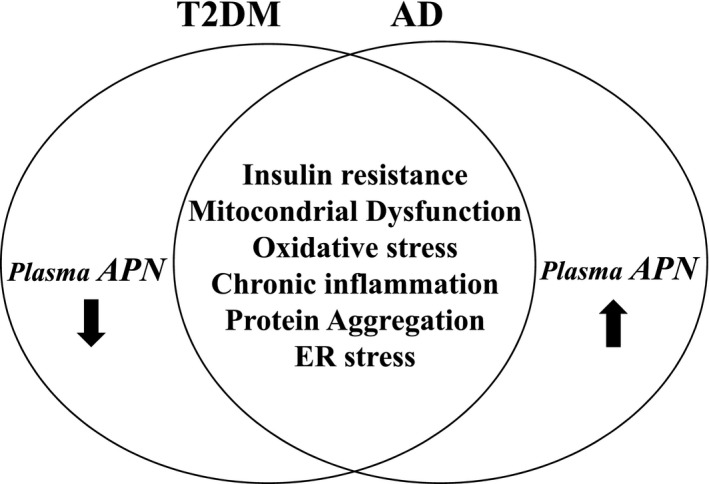
Differential alteration of plasma APN in T2DM and AD. T2DM and AD share a number of pathological features, such as insulin resistance, mitochondrial dysfunction, oxidative stress, chronic inflammation, protein aggregation, and endoplasmic reticulum stress. However, the level of plasma APN is decreased in T2DM, but increased in AD.
Recently, there has been a great interest in adiponectin (ANP) a benign adipocytokine that sensitizes the insulin receptor signaling pathway and suppresses inflammation. As expression of APN is decreased under conditions of general metabolic dysfunction, including obesity, T2DM, and cardiovascular diseases (Fig. 1),4 it is reasonable to speculate that ‘loss of function’ of APN might promote metabolic diseases and that restoration of APN signaling might be beneficial. Consistent with this idea, it has been shown that stimulation of APN signaling by recombinant APN or an APN receptor agonist is effective in the treatment of a mouse model of metabolic disorders.5, 6
Based on the overlapping pathologies between AD and T2DM, it is likely that plasma APN might also be reduced in AD. Indeed, APN has been shown to ameliorate neuropathological features in a mouse model of neurodegenerative disease.7, 8 Yet, in contrast, many studies have found that plasma APN is upregulated, rather than downregulated, in AD (Fig. 1).9, 10, 11, 12, 13 More recently, increased levels of plasma APN were found to be associated with the burden of amyloid deposits and degree of cognitive impairment in a prospective cohort study for the elderly,14 also suggesting that APN may instead promote neurodegeneration in AD.
The objective of this study is to discuss the potential mechanisms underlying the differential alterations of plasma APN between AD and T2DM. Further insights into the various mechanisms through which APN is involved in the pathogenesis of AD may provide clues from which to devise novel therapeutic strategies against AD and related neurodegenerative diseases.
APN actions in the nervous system
Compared to the abundant expression of APN in plasma, small amounts of APN exist in neuronal cells.15, 16 However, the low levels of APN in the brain and cerebrospinal fluid (CSF)15 may be compensated by the presence of two high‐affinity APN receptors, AdipoR1 and AdipoR2,17 in the brain.
Various molecules have been characterized in APN receptor signaling pathway in cell‐based studies. Among them, AMP‐activated protein kinase (AMPK) is the major kinase that plays a central role in biological effects, such as stimulation of insulin sensitivity, mitochondrial biogenesis, and oxidative metabolism in various types of cells (Fig. 2).18 Another important signaling molecule in APN receptor signaling pathway is GSK‐3β. it is of note that p38‐MAPK is situated upstream of GSK‐3β, inhibiting GSK‐3β activity by phosphorylation, and leading to stimulation of neurogenesis and antineurodegeneration in neuroblastoma cells7, 19 (Fig. 2). Furthermore, given the negative regulation of AMPK by GSK‐3β 20, a cross‐talk between the AMPK and GSK‐3β signaling pathways may be important. Indeed, we previously observed that inhibition of AMPK enhanced phosphorylation of GSK‐3β neuroblastoma cells.7 Thus, further studies are warranted to confirm these findings in the brain.
Figure 2.
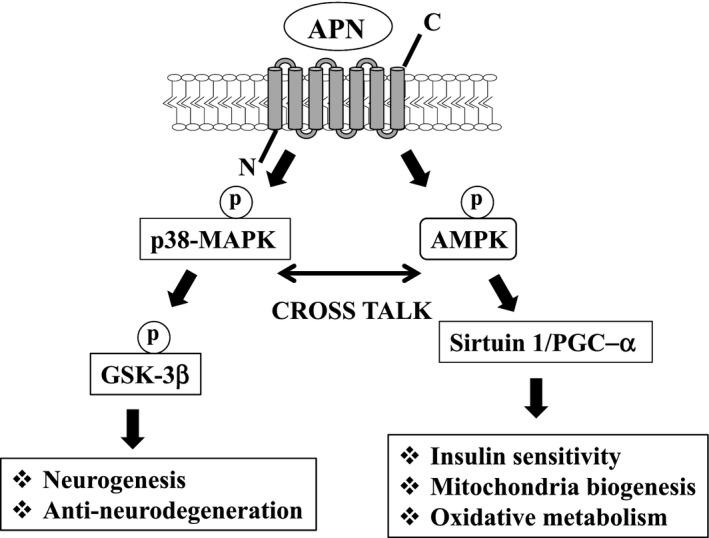
Schematic of APN signaling pathway. AMPK is the master kinase that regulates various intracellular signaling molecules, including sirtuin and PGC‐1α, leads to stimulation of insulin sensitivity, mitochondrial biogenesis, and oxidative metabolism. APN also activates p38‐MAPK, inhibiting GSK‐3β activity, leading to stimulation of neurogenesis and suppression of neurodegeneration. It is predicted that a crosstalk between the two pathways may be important for many biological effects in the nervous system.
Diverse actions of APN in the nervous system
As a member of a family of adipokines, including leptin, adipsin, and resistin, APN might be involved in regulation of energy balance and metabolism.21 AdipoR1 and AdipoR2 are both abundantly expressed in the hypothalamus, particularly in the arcuate nucleus and paraventricular hypothalamus.22 Intracerebroventricular administration of APN in a mouse model of T2DM was shown to decrease body weight, mostly through stimulating energy expenditure.23 Beyond energy regulation, APN may also play other roles in the nervous system. For instance, APN protected human neuroblastoma SH‐SY5Y cells against MPP+‐induced cytotoxicity24 and amyloid β‐induced neurotoxicity.25 In vivo, APN was protective against kainic acid‐induced excitotoxicity in the mouse hippocampus, a well‐established model of seizure.26 In addition, APN might be involved in regulation of neurogenesis because APN stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of the p38 mitogen‐activated protein kinase/glycogen synthase kinase 3β/β‐catenin signaling cascade.19 Physical exercise‐induced hippocampal neurogenesis is also mediated by APN,27 and APN exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus in mice.28
These findings show that APN has diverse effects in the nervous system, including neuroprotection, neurotrophic actions, and neurogenesis. Importantly, these effects might to some extent be attributed to modulation of insulin receptor signaling, given that APN can sensitize the insulin receptor signaling pathway.29 Moreover, APN may be important in suppression of neuroinflammation by glial cells. Thus, APN might be critical in normalizing the imbalance between M1 and M2 microglial polarization states,30 while globular APN induces a proinflammatory response in human astrocytes.31 Thus, further investigation of the in vivo effects of APN on glia, including microglia and astrocytes, is warranted.
APN ameliorates neurodegeneration in model mice
Given the diversity of its functions in the nervous system, APN may be beneficial in therapy for neurodegenerative disorders. In support of this view, APN ameliorated neuropathological features, such as protein aggregation and impaired motor activity, in a mouse model of α‐synucleinopathies.7 Osmotin, a plant homologue of APN, attenuates Aβ42‐induced neurotoxicity and tau hyperphosphorylation in the hippocampus of wild‐type mice,8 and it was recently shown that aged APN‐knockout mice have characteristics of brain insulin desensitization and development of an AD‐like pathology.32
APN may increase AD risk
Insights from in vivo and in vitro studies of APN strongly suggest a protective effect against AD and other neurodegenerative diseases, but the precise role of APN remains unclear. Thus, a clearer understanding of the biological actions of endogenous APN is required in the context of the normal aging brain, as well as in the pathogenesis of AD.
Increased level of plasma APN in AD
Accumulating evidence indicates that metabolic dysfunctions, such as T2DM, obesity, and atherosclerosis, increase the risk for AD and vascular dementia, and hypoadiponectinemia is a well‐characterized feature of metabolic syndrome.33 Therefore, it seems that plasma APN should be reduced in AD. Yet, paradoxically, studies in AD patients demonstrate an inverse relationship between APN and AD. Une and colleagues initially showed that the levels of plasma APN in mild cognitive impairment (MCI) (n = 18) and AD (n = 27) were significantly higher than those in normal controls (NC) (n = 28) (Table 1), and that the plasma and CSF levels of APN had a positive correlation.9 Consistent with these findings, the Framingham Heart Study undertook a prospective study of the contribution of biomarkers related to glucose homeostasis and inflammation to the risk of developing AD and all‐cause dementia in 840 dementia‐free participants (541 women and 299 men with a mean age of 72 years) showed that elevated APN was a predictor for all‐cause dementia and AD (Table 1).10 Notably, the increase in plasma APN in AD was significant in women, but not in men.10
Table 1.
Recent studies of plasma APN in patients with AD
| Study/Citation | Sample numbers | Plasma APN Level in AD | Other findings |
|---|---|---|---|
| Ma J et al. (2016) | NC = 91, AD = 91 | Increase | Insulin was increased, but leptin was decreased in AD compared to NC |
| Waragai M et al. (2016) | NC = 62, MCI = 64, AD = 63 | Increase | CSF_APN was decreased in AD compared to MCI and NC |
| Dukic L et al. (2016) | NC = 50, MCI = 48, AD = 70, VAD = 67 | No change | Similarly, Kallikrein‐ 6 and clustrin were no changes |
| Khemka VK et al. (2014) | NC = 60, AD = 60 | Increase | Insulin was increased, but leptin was unchanged |
| Teixeira AL et al. (2013) | NC = 51, MCI = 65, AD = 41 | Decrease | Follow‐up of MCI (n = 54) and NC (n = 43) did not show correlation of plasma APN with the disease progression |
| Warren MW et al. (2012) | NC = 198, AD = 148 | No change | Leptin was also unchanged |
| van Himbergen TM et al. (2012) | Prospective cohort project: dementia free (men: n = 299, women: n = 541, median age of 76 years) | Increase | APN levels were increased in all‐cause of dementia and AD in women |
| Une K et al. (2011) | NC = 28, MCI = 18, AD = 27 | Increase | CSF_APN was increased in MCI and in AD |
Increased levels of plasma APN in AD were confirmed by Khemka et al,11 Waragai et al.,12 and Ma et al.13, but Warren et al.34 and Dukic et al.35 did not find significant changes in plasma APN in AD patients, and lower plasma APN was found by Teixeira et al. (Table 1).36 The reasons for the discrepancies among these studies are unclear, but the APN level may be affected by multiple factors. Given that many AD patients have concomitant metabolic disorders,1 it is possible that plasma APN may fluctuate during coprogression of the disease.37 Furthermore, hypoadiponectinemia has been observed in alexithymia and tension‐type headache, in addition to metabolic disorders.38, 39 Thus, a study with a small sample size might be influenced by concurrent diseases or states that also affect APN levels.
Altered plasma APN is associated with neurodegenerative pathology
Given the diverse effects of APN on the nervous system, alterations in plasma APN might influence neuropathological changes in the brain. Indeed, it is well known that T2DM is independently associated with cognitive dysfunction and loss of hippocampus volume.40 Consistent with this, there is a significant decrease in hippocampus volume in patients with T2DM (n = 45) with low APN (Table 2).41 More recently, lower APN levels in T2DM (DM; n = 25, NC; n = 25) have been associated with lower gray matter volume and reduced cerebral glucose metabolism in temporal regions (Table 2).42 Thus, decreased plasma APN in T2DM might lead to degenerative changes in the hippocampus.
Table 2.
Association of the alteration of plasma APN with neuropathology
| Study/Citation | Sample numbers | Disease | Findings |
|---|---|---|---|
| Wennberg AM et al. (2016) | Nondemented (men: n = 328, women: n = 207) | AD | Correlation of high plasma APN with neuropathology (amyloid deposition and cognitive function) |
| Garcia‐Casares N et al. (2016) | T2DM (n = 25), NC (n = 25) | T2DM | Correlation of low plasma APN with hippocampal volume |
| Masaki T et al. (2012) | T2DM (n = 50) | T2DM | Correlation of low plasma APN with hippocampal volume |
On the other hand, a report derived from the Mayo Clinic Study of Aging, a large cohort study of aging and dementia (n = 535, aged ≥ 70 years without dementia) showed that plasma APN levels were associated with magnetic resonance imaging data of hippocampal and cortical volumes, fluorodeoxyglucose‐positron emission tomography (PET), amyloid‐PET, and cognitive outcomes, such as MCI diagnosis and neuropsychological test performance, suggesting that higher plasma APN may be associated with neuropathological features of AD, such as amyloid deposits and cognitive deficits.14
Taken together, an alteration of plasma APN, either a decrease or an increase, may lead to stimulation of neuropathology.
Gender difference in the association of APN with AD
Notably, the result of the Mayo Clinic study is consistent with those of the Framingham Heart Study, in which increased plasma APN levels were an independent risk factor for development of both all‐cause dementia and AD in women, but not in men.10, 14 One may predict that the gender difference of the association of APN with AD may be attributed to a hormonal difference, as APN and estrogen are inversely correlated and APN is increased in postmenopausal women.14, 43 Alternatively, women are likely to have more cognitive impairment than men at the same level of neuropathology.14, 44 Thus, a gender difference characterizes the relationship of increased plasma APN, leading to neurodegeneration in women with AD. Further investigations are warranted to determine the potential mechanisms behind the gender differences.
APN may promote neurodegeneration in AD through novel mechanisms
The mechanism by which increased expression of plasma APN, and presumably APN in the nervous system, promotes neurodegeneration in AD remains unknown. However, increasing evidence suggests that adaptation by APN to an insulin‐resistant state may be involved in the pathogenesis of AD.
Potential mechanism of upregulation of plasma APN in AD
Given that the network of insulin/IGF‐1‐APN signaling pathways is indispensable to the nervous system,45 upregulation of plasma APN may reflect a compensatory feedback response to abnormally reduced activity of insulin/IGF‐1 receptor signaling pathways in AD (Fig. 2). Indeed, it has been shown that the insulin signaling pathway is regulated by various feedback mechanisms. For instance, plasma insulin is upregulated in response to downregulation of insulin receptor signaling, resulting in insulin resistance,46 and APPL1, an adaptor protein containing a PH domain, PTB domain, and leucine zipper motif,47 has been increased in AD, perhaps in response to impaired insulin receptor signaling.48
An alternative, nonmutually exclusive possibility is that upregulation of plasma APN might instead be due to decreased activity of APN receptor signaling; in essence, APN resistance. Similarly to insulin and leptin resistance, APN resistance could be caused by dysregulation of signal transduction, ER stress, and inflammation.49 To our knowledge, APN receptor signaling functions in relation to neurodegeneration have not been examined, and studies of this critical issue in AD are required.
Discrepancy of expression patterns between APN and APN receptors
Whereas APN receptors are predominantly expressed in the hypothalamus,22 there seem to be extensive changes in APN levels in other brain regions in neurodegenerative diseases. Currently, the mechanism through which the expression pattern of APN is distinct from those of APN receptors remains unclear. In this regard, there are a few possibilities as follows. First, although it has generally been thought that APN is exclusively produced in adipocytes, a recent study suggests that expression of APN is induced in other tissues under the pathological conditions, such as skeletal muscle cells in T2D50 and aortic endothelial cells in cold storage.51 Therefore, it is not surprising that expression of APN is observed in degenerating neurons. A second possibility is that APN might have other signal transduction pathways in addition to AdipoR1 and ‐R2. For instance, it will be interesting to determine if T‐cadherin, which is abundantly expressed in brain52 and is known to bind APN,53 might affect expression of APN.
Insulin resistance may mediate APN‐related neuropathology
An increase in APN may compensate for compromised insulin receptor signaling, but various amyloidogenic proteins, including tau and α‐synuclein (α‐syn), might also gradually sequester APN, resulting in promotion of neurodegeneration. This concept is based on our recent observation that APN immunoreactivity is present in inclusion bodies, such as neurofibrillary tangles and Lewy bodies.7, 12 This raises a fundamental question regarding how secreted APN might interact with intracellular molecules such as tau and α‐syn. However, amyloid precursor protein (APP), another secreted molecule, is immunolocalized with Lewy bodies in PD brain,54 suggesting that there might be an as yet uncharacterized mechanism underlying this phenomenon. Thus, insulin resistance may upregulate APN, contributing to aggregation of tau in AD (Fig. 3). It is important to investigate how APN is involved in the formation of neurofibrillary tangles.
Figure 3.
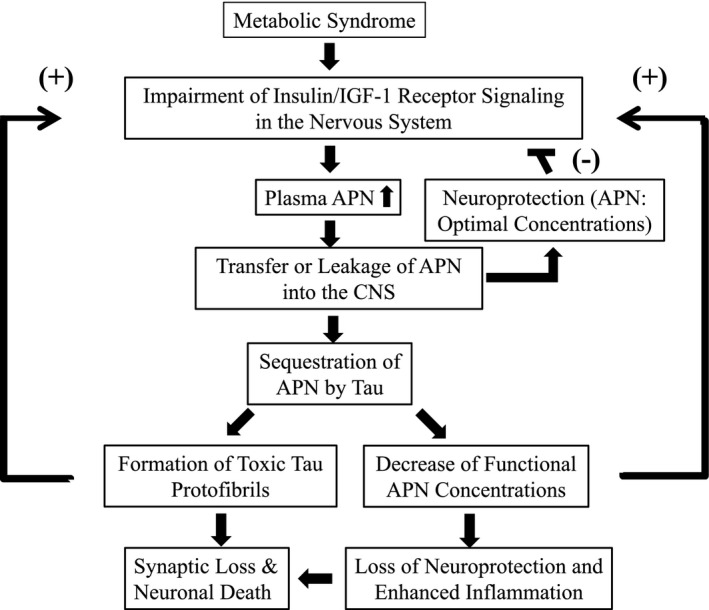
Hypothetical mechanism through which APN is involved in neuropathology in AD. Under metabolic syndrome conditions, AD is stimulated due to insulin resistance in the nervous system. In the early stage, plasma APN may be upregulated to compensate for compromised activity of the insulin receptor signaling pathway. APN may be transferred from plasma into the CNS in a regulated manner and ameliorates compromised activity of the insulin receptor signaling pathway. Thus, APN is thought to be neuroprotective through a negative feedback mechanism: (‐). However, during aging and progression of AD, APN may leak from plasma into the CNS, thereby being sequestered by tau and resulting in formation of toxic protofibrils of tau, leading to synaptic dysfunction and neuronal cell death (gain of function). Alternatively, sequestration of APN by tau results in decreased concentration and misfolding of APN, leading to downregulation of the insulin receptor signaling pathway. As a result, neurotrophic and neuroprotective activities of the insulin/APN receptor signaling network are lost, which is associated with increased neuroinflammation (loss of function). Thus, both ‘gain of function’ and ‘loss of function’ of APN may exacerbate the insulin receptor signaling positive feedback mechanism: (+), ultimately leading to synaptic dysfunction and neuronal cell death.
This proposed mechanism for the role of APN in the pathogenesis of AD is reminiscent of insulin degrading enzyme (IDE), a protease that digests both insulin and amyloid β (Aβ). A prevailing view is that IDE may be deprived by increased expression of insulin in response to compromised insulin receptor signaling, leading to Aβ accumulation and senile plaque formation in AD (Fig. 2).55 Alternatively, expression of IDE may be downregulated as IDE is a downstream target of the insulin receptor signaling pathway.56 In other words, insulin resistance may be situated upstream of the neurodegeneration cascade and adaptation to insulin resistance by APN may play a central part in the pathogenesis of AD, including formation of two major histopathological hallmarks of AD: senile plaques and neurofibrillary tangles.
The pathological lesions associated with neurodegeneration may be steps in a cascade leading to synaptic dysfunction and ultimately to neuronal cell death, the common denominators of neurodegenerative disease. As such, toxic aggregates of amyloidogenic proteins, such as Aβ, tau, and α‐syn, and particularly protofibril forms, might be implicated in this process.57 Based on the colocalization of APN with tau,12, it is also possible that APN may be sequestered by tau into neurofibrillary tangles and misfolding of APN might be stimulated thereby. Under such neurodegenerative conditions, it is predicted that the insulin–APN signal transduction network may result in downregulation of neuroprotective and neurotrophic activities of neurons, leading to synaptic dysfunction and neuronal cell death. Further mechanistic studies of the interaction of APN and tau are warranted to examine this possibility.
Furthermore, given the importance of APN as a negative regulator of inflammation, failure to suppress neuroinflammation by APN may further exacerbate the insulin–APN signal transduction network (Fig. 2). Collectively, APN alone takes on dual properties of ‘gain of function’ and ‘loss of function’, leading to neurodegeneration (Fig. 2). Therefore, it is intriguing to speculate that fluctuation of APN might act as a driving force in the pathogenesis of AD.
Leakage of plasma APN may lead to neurodegeneration
Because the integrity of the blood–brain barrier (BBB) gradually decreases in aging58, 59 and amyloid deposition in cerebral blood vessels is almost universal in AD, leakage of plasma protein has long been postulated as causative of disease.60 In this context, leakage of plasma APN into the brain parenchyma may result in sequestration of amyloidogenic proteins, such as tau and α‐syn, leading to stimulation of neurodegeneration. Naturally, such a mechanism may be involved in vascular dementia that may cause BBB dysfunction, in which APN may play a major role60. Thus, APN could be a dangerous neurodegenerative molecule, and this might explain why little APN is expressed in the nervous system, compared to the strong expression of APN in serum.61 Given the tendency for higher plasma APN in women compared to men,61 we predict that the gender difference regarding the correlation of plasma APN with neuropathology61 may be at least in part attributable to the decrease in BBB integrity in aging, in addition to other mechanisms, including hormonal differences between men and women.
Differential action of APN in rodents and humans
There is a striking difference in APN actions between mouse models of neurodegeneration and human brains, with respect to neurodegenerative disease. APN appears to be neuroprotective in brains of mice7, 8, whereas it may promote neurodegeneration in the human brain.14 The mechanisms underlying these differential actions of APP in neurodegeneration is a central question in neurodegenerative research, considering that there are differences in neuropathological features in mouse and human brains in various aspects of neurodegenerative processes.
Difference in aging‐associated protein aggregation between rodents and humans
Postmenopausal aging in women is distinct from that in other vertebrates, and this may be relevant to the differential effects of APN in humans and mice. Based on the absence of neurohistopathologies, such as Lewy bodies and neurofibrillary tangles, in brains of neurodegenerative mice models, the degree of protein aggregation‐related pathology in aging mice may be much milder than that in humans. Activities of the protein degradation systems, such as autophagy and the ubiquitin proteasome system, are compromised in aging62, 63, which suggests that clearance of protein aggregation in aging might be more efficient in mice than in humans. From this perspective, it is probable that toxic gain of function of APN through interaction with tau may be specific to human aging, whereas loss of neuroprotective and neurotrophic functions of APN due to a decreased activity of the insulin/APN receptors signaling network might be common between humans and mice.
Further impact of gender on APN in animal models
Gender differences in APN activity, especially in the circulation, should clearly be taken into account in studies of APN function and influence on neurodegeneration. However, all studies of APN actions in neurodegenerative diseases have been performed in male mice to exclude the effects of the estrous cycle on behavioral and psychiatric states, including mood, anxiety, learning, and memory.7, 8, 32, 64 Thus, a study is required for comparison of the effects of APN in male and female mice.
APN as a novel target for therapies for AD
Despite numerous clinical trials in AD directed toward protein aggregation, oxidative stress, and inflammation, which are all molecular targets implicated in the pathogenesis,45 therapeutic outcomes have been unsatisfactory. Given that metabolic dysfunction is central to AD, ‘anti‐insulin resistance’ has recently been proposed as a paradigm in therapy for AD and related disorders. Within this framework, the mechanistic actions of APN in AD could be considered. In support of this idea, it is noteworthy that polymorphisms of the APN gene have recently been linked to sporadic AD.65, 66
APN‐based therapeutic strategy against AD
The dual neuroprotective and neurodegenerative properties of APN in AD must be considered in development of a therapeutic strategy against AD. First, if stimulation of the APN receptor signaling pathway is protective against neurodegeneration, the APN receptor could be targeted to improve insulin resistance, as in metabolic disorders.6 One candidate, a small‐molecule APN receptor agonist, AdipoRon, may be effective for stimulation of insulin signaling,6 although its ability to cross the blood‐brain barrier (BBB) has yet to be shown. Alternatively, other molecules that sensitize insulin receptor signaling, such as glucagon‐like peptide 1 (GLP‐1), could be targeted.67 In fact, clinical studies are underway to evaluate the efficacy of a GLP‐1 analogue in mild‐to‐moderate AD.45 In parallel, suppression of the neurodegenerative action of APN is required. Inhibition of tau aggregation may be an efficient strategy to inhibit sequestration of APN by tau, and a clinical study of tau immunization for progressive supranuclear palsy is ongoing.68 However, stimulating endogenous production of APN using compounds such as PPAR‐γ agonists,69 acetylcholinesterase inhibitors,70 and vitamin E71 may be detrimental in terms of the toxic gain of function of APN.
Given that plasma APN might leak into parenchyma through defective brain vascular structure and contribute to neurodegeneration, preservation of the integrity of the BBB is another important strategy. Thus, it is worth noting that antihypertensive agents, such as angiotensin‐converting enzyme (ACE) inhibitors, have been shown to be beneficial as therapy for AD,72 as prevention of atherosclerosis may enhance the integrity of the BBB. However, caution is necessary because treatment with ACE inhibitors upregulates APN expression in serum.73, 74 Overall, combined treatment with an APN receptor agonist and tau immunotherapy may provide greater therapeutic efficacy. Indeed, combination therapy of anti‐insulin resistance with amyloid immunotherapy has been proposed for AD and related neurodegenerative diseases.45
APN as an early biomarker of AD
Finally, it is increasingly clear that early treatment is essential to protect against AD.45 In this regard, APN could potentially serve as a marker of early‐stage disease and perhaps of disease progression. Given that disease‐modifying therapies should be initiated at earlier stages of disease pathogenesis,7 it will be interesting to determine whether APN can play a role in evaluating therapeutic effects in AD.
To date, only two studies have evaluated the association of CSF APN and AD. Une et al. found increased CSF APN in AD and MCI patients, suggesting that increased APN in CSF may be derived from increased APN in serum9, although it is yet to be determined if this is physiological or due to decreased activity of BBB in aging or under neurodegenerative conditions. In contrast, Waragai et al. found that CSF APN was reduced in AD patients.12 The authors suggested that APN might have been sequestered to neurofibrillary tangles, similar to sequestration of Aβ42 into senile plaques in CSF of AD.12 Considering that APN is a potential biomarker of AD, further studies are warranted to clarify the relationship between plasma and CNS APN levels in both health and disease.
Conclusions
A recent finding of an association of elevated plasma APN with degenerative neuropathology, including amyloid deposits and severity of dementia severity in the elderly14, together with accumulating reports of upregulation of plasma APN in AD suggests that a gain of function of APN may be involved in development of AD. It is possible that stimulation of protein aggregation and toxic fibril formation of tau may exacerbate insulin resistance (Fig. 3). On the other hand, loss of neuroprotective and neurotrophic functions of the insulin/APN receptor sigsignaling network being accompanied with increased neruinflammation may also exacerbate insulin resistance (Fig. 3). Thus, both aspects of ‘gain of function’ and ‘loss of function’ of APN in the pathogenesis of AD may ultimately lead to synaptic dysfunction and neuronal cell death (Fig. 3). In concert with other adipocytokines, such as leptin75, a complicated mode of APN may act as a driving force in AD pathogenesis.
Such a molecular action of APN in AD is in contradiction to the loss of function of APN in hypoadiponectinemia in metabolic disorders, including obesity and T2DM. Therefore, a new therapeutic strategy reflecting the toxic effects of APN in AD may be required that is distinct from conventional treatment for metabolic disorders. In this regard, we suggest that a combination of an APN receptor agonist and tau immunotherapy may be a viable strategy. In addition, APN may be useful as an early biomarker of AD. This is of particular interest in efforts to develop disease‐modifying therapies for AD. Collectively, APN may be mechanistically, therapeutically, and diagnostically important in AD.
Author Contributions
MW, GH, and MH wrote the manuscript. EM, SS, TT, YT, and KS discussed the ideas in the manuscript. All authors have read and approved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported in part by a Basic Science Research B 25290019 grant‐in aid (to MH) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Funding Statement
This work was funded by Ministry of Education, Culture, Sports, Science, and Technology, Japan grant Basic Science Research B 25290019.
Contributor Information
Masaaki Waragai, Email: waragai@kk.iij4u.or.jp.
Makoto Hashimoto, Email: hashimoto-mk@igakuken.or.jp.
References
- 1. Cai H, Cong WN, Ji S, et al. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders. Curr Alzheimer Res 2012;9:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frisardi V, Solfrizzi V, Seripa D, et al. Metabolic‐cognitive syndrome: a cross‐talk between metabolic syndrome and Alzheimer's disease. Ageing Res Rev 2010;9:399–417. [DOI] [PubMed] [Google Scholar]
- 3. Walker JM, Harrison FE. Shared neuropathological characteristics of obesity, type 2 diabetes and Alzheimer's disease: Impacts on cognitive decline. Nutrients 2015;7:7332–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004;24:29–33. [DOI] [PubMed] [Google Scholar]
- 5. Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE‐deficient mice from atherosclerosis. J Biol Chem 2003;278:2461–2468. [DOI] [PubMed] [Google Scholar]
- 6. Okada‐Iwabu M, Yamauchi T, Iwabu M, et al. A small‐molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013;503:493–499. [DOI] [PubMed] [Google Scholar]
- 7. Sekiyama K, Waragai M, Akatsu H, et al. Disease‐modifying effect of adiponectin in model of alpha‐synucleinopathies. Ann Clin Transl Neurol 2014;1:479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ali T, Yoon GH, Shah SA, et al. Osmotin attenuates amyloid beta‐induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci Rep 2015;5:11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Une K, Takei YA, Tomita N, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer's disease. Eur J Neurol 2011;18:1006–1009. [DOI] [PubMed] [Google Scholar]
- 10. van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all‐cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch Neurol 2012;69:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khemka VK, Bagchi D, Bandyopadhyay K, et al. Altered serum levels of adipokines and insulin in probable Alzheimer's disease. J Alzheimers Dis 2014;41:525–533. [DOI] [PubMed] [Google Scholar]
- 12. Waragai M, Adame A, Trinh I, et al. Possible Involvement of adiponectin, the anti‐diabetes molecule, in the Pathogenesis of Alzheimer's disease. J Alzheimers Dis 2016;52:1453–1459. [DOI] [PubMed] [Google Scholar]
- 13. Ma J, Zhang W, Wang HF, et al. Peripheral blood adipokines and insulin levels in patients with Alzheimer's disease: A replication study and meta‐analysis. Curr Alzheimer Res 2016;13:223–233. [DOI] [PubMed] [Google Scholar]
- 14. Wennberg AM, Gustafson D, Hagen CE, et al. Serum adiponectin levels, neuroimaging, and cognition in the mayo clinic study of aging. J Alzheimers Dis 2016;53:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebinuma H, Miida T, Yamauchi T, et al. Improved ELISA for selective measurement of adiponectin multimers and identification of adiponectin in human cerebrospinal fluid. Clin Chem 2007;53:1541–1544. [DOI] [PubMed] [Google Scholar]
- 16. Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 2008;582:74–80. [DOI] [PubMed] [Google Scholar]
- 17. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–769. [DOI] [PubMed] [Google Scholar]
- 18. Kadowaki T, Yamauchi T. Adiponectin receptor signaling: a new layer to the current model. Cell Metab 2011;13:123–124. [DOI] [PubMed] [Google Scholar]
- 19. Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen‐activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK‐3beta)/beta‐catenin signaling cascade. J Biol Chem 2011;286:44913–44920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki T, Bridges D, Nakada D, et al. Inhibition of AMPK catabolic action by GSK3. Mol Cell 2013;50:407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol 2014;13:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP‐activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007;6:55–68. [DOI] [PubMed] [Google Scholar]
- 23. Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med 2004;10:524–529. [DOI] [PubMed] [Google Scholar]
- 24. Jung TW, Lee JY, Shim WS, et al. Adiponectin protects human neuroblastoma SH‐SY5Y cells against MPP+‐induced cytotoxicity. Biochem Biophys Res Commun 2006;343:564–570. [DOI] [PubMed] [Google Scholar]
- 25. Chan KH, Lam KS, Cheng OY, et al. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid‐beta neurotoxicity. PLoS ONE 2012;7:e52354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeon BT, Shin HJ, Kim JB, et al. Adiponectin protects hippocampal neurons against kainic acid‐induced excitotoxicity. Brain Res Rev 2009;61:81–88. [DOI] [PubMed] [Google Scholar]
- 27. Yau SY, Li A, Hoo RL, et al. Physical exercise‐induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA 2014;111:15810–15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang D, Wang X, Lu XY. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology 2016;157:2853–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–451. [DOI] [PubMed] [Google Scholar]
- 30. Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti‐inflammatory phenotype. J Biol Chem 2010;285:6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wan Z, Mah D, Simtchouk S, et al. Globular adiponectin induces a pro‐inflammatory response in human astrocytic cells. Biochem Biophys Res Commun 2014;446:37–42. [DOI] [PubMed] [Google Scholar]
- 32. Ng RC, Cheng OY, Jian M, et al. Chronic adiponectin deficiency leads to Alzheimer's disease‐like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol Neurodegener 2016;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Funahashi T, Matsuzawa Y. Hypoadiponectinemia: a common basis for diseases associated with overnutrition. Curr Atheroscler Rep 2006;8:433–438. [DOI] [PubMed] [Google Scholar]
- 34. Warren MW, Hynan LS, Weiner MF. Lipids and adipokines as risk factors for Alzheimer's disease. J Alzheimers Dis 2012;29:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dukic L, Simundic AM, Martinic‐Popovic I, et al. The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. Clin Biochem 2016;49:213–218. [DOI] [PubMed] [Google Scholar]
- 36. Teixeira AL, Diniz BS, Campos AC, et al. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer's disease. Neuromolecular Med 2013;15:115–121. [DOI] [PubMed] [Google Scholar]
- 37. Besser LM, Gill DP, Monsell SE, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord 2014;28:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Honkalampi K, Viinamaki H, Niskanen L, et al. Reduced serum adiponectin levels in alexithymia. NeuroImmunoModulation 2014;21:234–239. [DOI] [PubMed] [Google Scholar]
- 39. Domingues RB, Duarte H, Rocha NP, Teixeira AL. Reduced serum levels of adiponectin in tension‐type headache. Clin Neurol Neurosurg 2015;131:82–85. [DOI] [PubMed] [Google Scholar]
- 40. Jack CR Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology 1998;51:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masaki T, Anan F, Shimomura T, et al. Association between hippocampal volume and serum adiponectin in patients with type 2 diabetes mellitus. Metabolism 2012;61:1197–1200. [DOI] [PubMed] [Google Scholar]
- 42. Garcia‐Casares N, Garcia‐Arnes JA, Rioja J, et al. Alzheimer's like brain changes correlate with low adiponectin plasma levels in type 2 diabetic patients. J Diabetes Complications 2016;30:281–286. [DOI] [PubMed] [Google Scholar]
- 43. Leung KC, Xu A, Craig ME, et al. Adiponectin isoform distribution in women–relationship to female sex steroids and insulin sensitivity. Metabolism 2009;58:239–245. [DOI] [PubMed] [Google Scholar]
- 44. Barnes LL, Wilson RS, Bienias JL, et al. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 2005;62:685–691. [DOI] [PubMed] [Google Scholar]
- 45. Takamatasu Y, Ho G, Koike W, Sugama S, Takenouchi T, Waragai M, Wei J, Sekiyama K, Hashimoto M. Combined immunotherapy with ‘anti‐insulin resistance therapy’ as a novel therapeutic strategy against neurodegenerative diseases. NPJ Parkinson's disease 2017; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brands MW, Hall JE. Insulin resistance, hyperinsulinemia, and obesity‐associated hypertension. J Am Soc Nephrol 1992;3:1064–1077. [DOI] [PubMed] [Google Scholar]
- 47. Mao X, Kikani CK, Riojas RA, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 2006;8:516–523. [DOI] [PubMed] [Google Scholar]
- 48. Ogawa A, Yamazaki Y, Nakamori M, et al. Characterization and distribution of adaptor protein containing a PH domain, PTB domain and leucine zipper motif (APPL1) in Alzheimer's disease hippocampus: an immunohistochemical study. Brain Res 2013;1494:118–124. [DOI] [PubMed] [Google Scholar]
- 49. Hosoi T, Ozawa K. Possible pharmacological approach targeting endoplasmic reticulum stress to ameliorate leptin resistance in obesity. Front Endocrinol (Lausanne) 2016;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delaigle AM, Senou M, Guiot Y, et al. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: In vivo and in vitro studies. Diabetologia 2006;49:1311–1323. [DOI] [PubMed] [Google Scholar]
- 51. Ebner A, Poitz DM, Alexiou K, Deussen A. Secretion of adiponectin from mouse aorta and its role in cold storage‐induced vascular dysfunction. Basic Res Cardiol 2013;108:390. [DOI] [PubMed] [Google Scholar]
- 52. Takeuchi T, Misaki A, Liang SB, et al. Expression of T‐cadherin (CDH13, H‐Cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem 2000;74:1489–1497. [DOI] [PubMed] [Google Scholar]
- 53. Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol 2012;165:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arai H, Lee VM, Hill WD, et al. Lewy bodies contain beta‐amyloid precursor proteins of Alzheimer's disease. Brain Res 1992;585:386–390. [DOI] [PubMed] [Google Scholar]
- 55. Xie L, Helmerhorst E, Taddei K et al. Alzheimer's beta‐amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci 2002; 22: RC221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao L, Teter B, Morihara T, et al. Insulin‐degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci 2004;24:11120–11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sekiyama K, Takamatsu Y, Koike W, et al. Insight into the dissociation of behavior from histology in Synucleinopathies and in related neurodegenerative diseases. J Alzheimer's Dis 2016;52:831–841. [DOI] [PubMed] [Google Scholar]
- 58. Hardy JA, Mann DM, Wester P, Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging 1986;7:489–502. [DOI] [PubMed] [Google Scholar]
- 59. Mooradian AD. Effect of aging on the blood‐brain barrier. Neurobiol Aging 1988;9:31–39. [DOI] [PubMed] [Google Scholar]
- 60. Kawai M, Kalaria RN, Harik SI, Perry G. The relationship of amyloid plaques to cerebral capillaries in Alzheimer's disease. Am J Pathol 1990;137:1435–1446. [PMC free article] [PubMed] [Google Scholar]
- 61. Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226–228. [DOI] [PubMed] [Google Scholar]
- 62. Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 2015;84:39–49. [DOI] [PubMed] [Google Scholar]
- 63. Saez I, Vilchez D. The mechanistic links between proteasome activity. Aging and age‐related diseases. Curr Genomics 2014;15:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim H, Son J, Yoo H, et al. Effects of the female estrous cycle on the sexual behaviors and ultrasonic vocalizations of male C57BL/6 and autistic BTBR T+ tf/J mice. Exp Neurobiol 2016;25:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barber RC, Phillips NR, Tilson JL, et al. Can genetic analysis of putative blood Alzheimer's disease biomarkers lead to identification of susceptibility loci? PLoS ONE 2015;10:e0142360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li W, Yu Z, Hou D, et al. Relationship between Adiponectin gene polymorphisms and late‐onset Alzheimer's disease. PLoS ONE 2015;10:e0125186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holscher C. Potential role of glucagon‐like peptide‐1 (GLP‐1) in neuroprotection. CNS Drugs 2012;26:871–882. [DOI] [PubMed] [Google Scholar]
- 68. Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer's disease. Trends Mol Med 2015;21:394–402. [DOI] [PubMed] [Google Scholar]
- 69. Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR gamma 2, but not C/EBP alpha‐evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun 2003;308:933–939. [DOI] [PubMed] [Google Scholar]
- 70. Pakaski M, Feher A, Juhasz A, et al. Serum adipokine levels modified by donepezil treatment in Alzheimer's disease. J Alzheimers Dis 2014;38:371–377. [DOI] [PubMed] [Google Scholar]
- 71. Gray B, Swick J, Ronnenberg AG. Vitamin E and adiponectin: proposed mechanism for vitamin E‐induced improvement in insulin sensitivity. Nutr Rev 2011;69:155–161. [DOI] [PubMed] [Google Scholar]
- 72. Savaskan E. The role of the brain renin‐angiotensin system in neurodegenerative disorders. Curr Alzheimer Res 2005;2:29–35. [DOI] [PubMed] [Google Scholar]
- 73. Furiya Y, Ryo M, Kawahara M, et al. Renin‐angiotensin system blockers affect cognitive decline and serum adipocytokines in Alzheimer's disease. Alzheimers Dement 2013;9:512–518. [DOI] [PubMed] [Google Scholar]
- 74. Fontana V, de Faria AP, Oliveira‐Paula GH, et al. Effects of angiotensin‐converting enzyme inhibition on leptin and adiponectin levels in essential hypertension. Basic Clin Pharmacol Toxicol 2014;114:472–475. [DOI] [PubMed] [Google Scholar]
- 75. Tezapsidis N, Johnston JM, Smith MA, et al. Leptin: a novel therapeutic strategy for Alzheimer's disease. J Alzheimers Dis 2009;16:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]


