Abstract
Introduction:
Anemia is a potential adverse effect of phlebotomy during participation in research. Clinical studies of acute HIV infection (AHI) require frequent phlebotomy to maximize scientific yield, but this participant population may also be at increased risk for anemia and other adverse events.
Objective:
The objective of this study was to describe baseline and longitudinal hemoglobin changes among participants with AHI.
Methods:
Participants with AHI (n = 202) were enrolled in a prospective cohort study in Thailand. AHI was diagnosed using pooled nucleic acid testing and sequential HIV antibody immunoassays. Antiretroviral therapy was initiated on enrollment. During 48 weeks of study participation, a total of 629 mL of blood was drawn over 14 visits. Hemoglobin levels were measured serially, and abnormalities were graded using the Division of AIDS (National Institute of Allergy and Infectious Diseases) adverse event table.
Results:
AHI was diagnosed at a median of 18 days after infection. Mean hemoglobin at enrollment of male participants was 14.8 g/dL, and for females, it was 13.0 g/dL. Over 48 weeks, there was a mean increase of 0.2 g/dL among men (P = 0.01) and a decrease of 0.7 g/dL among women (P = 0.03). The overall prevalence of anemia was low, with 7 (3.5%) of 202 fulfilling grade 1 or 2 anemia criteria.
Conclusions:
Anemia was rare after frequent phlebotomy in research participants with AHI, before and after antiretroviral therapy. Given that the blood volume drawn from this study did not pose substantial clinical risk, increasing the volume of blood drawn for research purposes in acute HIV-infected research participants could be considered for future studies.
Key Words: research risk, acute HIV infection, frequent blood sampling, anemia, phlebotomy
INTRODUCTION
Blood sampling is an essential tool for participant assessment during clinical research.1,2 However, the effect of phlebotomy on the hemoglobin levels of research participants is an issue of concern to potential trial participants, researchers, and institutional review boards (IRBs). Ethical concerns may arise when the number of blood drawn or the cumulative blood volume is perceived to be high.
Anemia, defined as a low blood hemoglobin concentration, has been linked to frequent blood sampling in hospitalized patients and reported to be common in HIV infection.1–4 Anemia in HIV can be caused by the virus itself, malnutrition, malignancies, infections, hemolysis and medications such as zidovudine.5
Limits in blood sampling for research are generally guided by the policies of IRBs. The United States Office of Human Research Protection published a guideline for blood sampling volume in clinical research, but the guideline applies only to the research deemed to have no more than minimal risk to participants.
Scarce data are available describing the hemoglobin level and effect of frequent phlebotomy for research of HIV-infected individuals in acute HIV infection (AHI), who can have anemia related directly to their HIV disease.6 It is important to optimize the amount of blood that can be safely collected in this population to maximize the scientific knowledge gained from research participation. The primary objective of this study was to describe baseline hemoglobin levels and longitudinal changes in hemoglobin among participants in a cohort study of AHI in Bangkok, Thailand. Findings of this study could inform guidelines regarding safe blood volume limits when conducting research in acutely HIV-infected populations.
MATERIALS AND METHODS
Study Population
The SEARCH010/RV254 (clinicaltrials.gov NCT00796146) is an ongoing longitudinal cohort of participants with AHI in Thailand. The study uses pooled nucleic acid testing, fourth generation HIV antigen/antibody immunoassay (IA), and sequential less sensitive HIV antibody IA to identify individuals with AHI in a voluntary counseling and testing clinic as previously described.7,8 Individuals are recruited into the study if they have either a nonreactive fourth generation IA and a positive nucleic acid testing or a reactive fourth generation IA and a nonreactive second or third generation IA. Participants are offered immediate initiation of antiretroviral therapy (ART) after enrollment. The study was approved by the IRBs of Chulalongkorn University in Thailand and the Walter Reed Army Institute of Research in the United States.
This analysis included all participants who enrolled in the cohort between May 2009 and September 2015, initiated ART during AHI (Fiebig stages I–V), and had hemoglobin assessment at baseline and after 48 weeks of study participation.
Definition and Grading of Hemoglobin
Hemoglobin was measured using a Sysmex XN-1000 (Sysmex Corporation, Kobe, Japan) automated blood cell analyzer. Severity of anemia was described according to the grading tables described by the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases.9 In men, anemia was defined as a hemoglobin level equal or below 10.9 g/dL and graded as mild (grade 1, 10.0–10.9 g/dL), moderate (grade 2, 9.0 to <10.0 g/dL), severe (grade 3, 7.0 to <9.0 g/dL), or potentially life threatening (grade 4, <7.0 g/dL). For adult women, hemoglobin equal to or below 10.4 g/dL was considered anemic and graded as mild (grade 1, 9.5–10.4 g/dL), moderate (grade 2, 8.5 to <9.5 g/dL), severe (grade 3, 6.5 to <8.5 g/dL), or potentially life threatening (grade 4, <6.5 g/dL).
Blood Sampling Volume
According to the SEARCH010/RV254 schedule of events, there are a total of 14 visits from week 0 to week 48 with a cumulative blood draw volume of 629 mL. Most of the blood draws occur during the first 4 months of study participation. For the purpose of this analysis, week 0 was considered to include phlebotomy at both the screening and enrollment visits, whereas week 2 includes blood draws conducted on days 2, 3, 5, 7, and 10. We collected 56 mL at week 0, a combined blood volume of 155 mL at the end of week 2, 56 mL at week 4, 54 mL at week 8, 57 mL at week 12, 44 mL at week 16, 44 mL at week 20, 59 mL at week 24, 44 mL at week 36, and 60 mL at week 48.
Statistical Methods
Hemoglobin at enrollment and subsequent changes from baseline were described by mean and SD, stratified by sex. We used a generalized estimating equations model with an exchangeable correlation matrix to assess changes from baseline. Significance was determined by a P value <0.05 for all calculations. Analyses were performed using Stata Statistical Software: Release 13. (StataCorp LP, College Station, TX). Figures were generated with Prism version 6.02 for Windows (GraphPad Software, La Jolla, CA).
RESULTS
Study Population Characteristics at Baseline
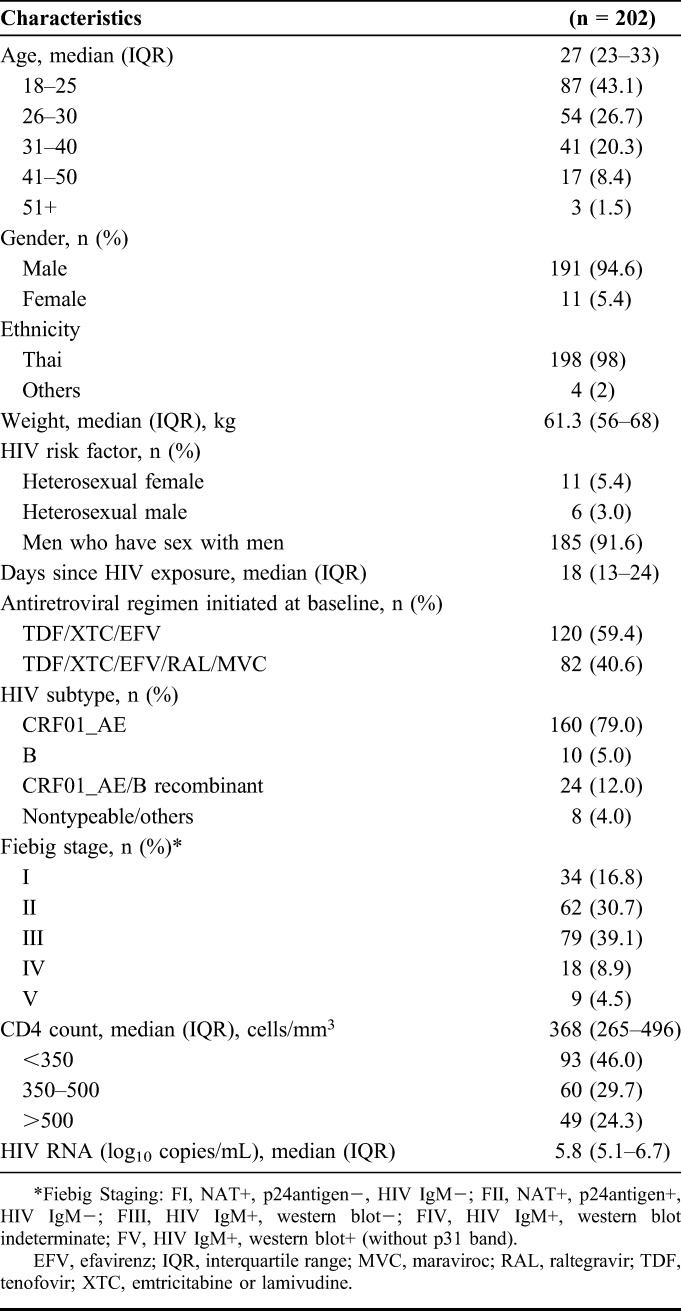
We enrolled 213 individuals with AHI between May 2009 and September 2015. Eleven participants were excluded from the analysis for the following reasons: 3 missed the week 48 visit, 3 withdrew consent before week 48, 2 participants were no longer in AHI at the time of enrollment (Fiebig VI), and 2 participants did not start ART at week 0. A total of 202 participants were included in the analysis. Table 1 describes the characteristics of study participants at enrollment. On enrollment, all participants initiated ART that included efavirenz, tenofovir, and either lamivudine or emtricitabine. A subgroup of 82 participants (40.6%) received an intensified regimen that additionally included raltegravir and maraviroc for the first 24 weeks of therapy.
TABLE 1.
Study Population Characteristics at Baseline
Hemoglobin During AHI
On diagnosis of AHI (week 0), the mean hemoglobin level of the cohort was 14.7 g/dL (SD 1.3). The mean hemoglobin level for men was 14.8 g/dL (SD 1.26), and for women, it was 13.0 g/dL (SD 1.42). Mean hemoglobin levels at baseline for both male and female participants were above the threshold for anemia. The prevalence of low hemoglobin was 0.5% at week 0 with only one participant having grade 1 (mild) anemia.
Longitudinal Assessments of Hemoglobin
Mean hemoglobin levels during longitudinal study participation are depicted in Figure 1A. The mean hemoglobin level of study participants never fell below the threshold for DAIDS adverse event reporting of anemia for men or women.
FIGURE 1.
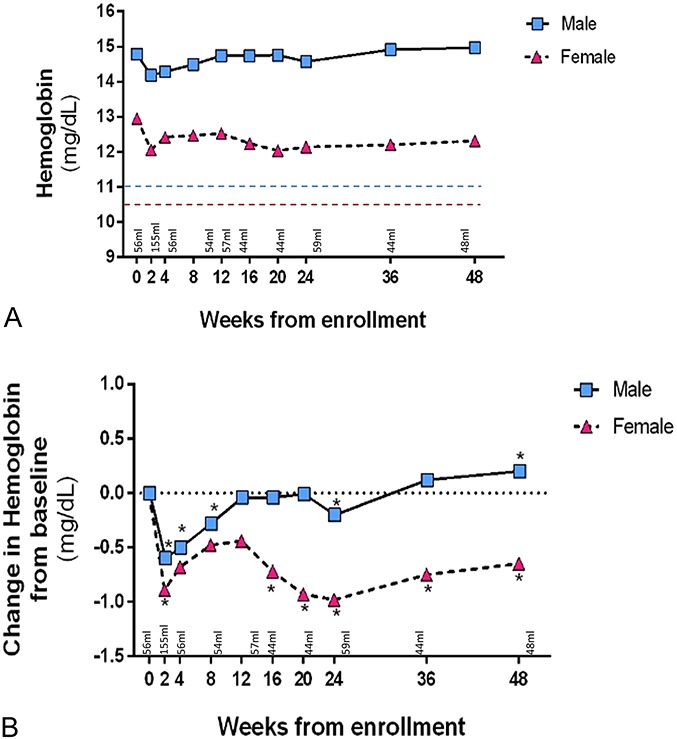
A, Mean hemoglobin level and B, Change in hemoglobin from baseline. DAIDS cut-off for low hemoglobin adverse event for men is 10.9 g/dL (blue line) and 10.4 g/dL for women (red line). P value from generalized estimating equations (GEE). Statistically significant differences (P < 0.05) marked with asterisk (*).
Mean changes in hemoglobin as compared to baseline are summarized for each sex in Figure 1B. In men, hemoglobin levels decreased significantly from baseline at week 2, week 4, and week 8, but this was no longer observed by weeks 12, 16, and 20. At week 24, the mean hemoglobin again had a significant decrease. Beginning at week 36, we observed a trend of rising hemoglobin, and by week 48, the mean hemoglobin of the study population had increased above baseline (+0.2 g/dL, P = 0.01).
By contrast, the women showed a significant decrease in hemoglobin levels throughout the study period. Although there was a recovery of hemoglobin drop from week 2 onwards, by week 48, the hemoglobin was significantly lower than the baseline value (−0.7 g/dL, P = 0.03, Fig. 1B).
Prevalence and Incidence of Anemia
Anemia was uncommon in this cohort, with only 1 prevalent case (0.5%) at the time of study enrollment and 6 incident cases (3.0%) during 48 weeks of observation. All cases were grade 1 or 2 according to DAIDS criteria. The prevalence of grade 1 or 2 anemia during specific visits ranged from 0.5% to 1.5%. Among 11 women, 2 (18.2%) experienced grade 2 anemia during follow-up and 1 (9.1%) experienced grade 1 anemia. By contrast, only 4 of 193 (2.1%) men experienced anemia, and all were grade 1 events.
DISCUSSION
Our findings suggest that anemia is rare during AHI. The mean hemoglobin levels observed during AHI among both male and female participants were above the criteria used to diagnose anemia. The mean hemoglobin level of our cohort was comparable to previously published normal values among healthy Thai volunteer blood donors, which include mean and SDs of 14.0 (±0.5) g/dL for men and 13.4 (±1.1) g/dL for women.10
We demonstrated that repeated blood draws after the local blood donation guidelines did not have lasting or clinically significant adverse effects on hemoglobin levels of participants. Hemoglobin declined in the first few weeks after study enrollment and subsequently improved. The decrease in hemoglobin was reversible as evidenced by a return to baseline levels beginning at week 36. A statistically significant drop in mean hemoglobin from baseline was noted at week 2, but the population mean hemoglobin remained within the normal range and did not satisfy WHO criteria for the diagnosis of anemia or DAIDS criteria for grade I adverse event classification.11 Symptoms of anemia usually manifest when anemia is severe (7.0–8.0 g/dL), and no participant reached this low level.12 After 48 weeks, hemoglobin actually increased compared with baseline in male participants despite serial phlebotomy.
Reduced hemoglobin level was commonly found in women. This may be related to the physiologic predisposition to anemia due to menstruation.13 The mean baseline hemoglobin level of women in our cohort was within the normal range of hemoglobin levels for nonpregnant, nonlactating women in Thailand.14 Compared with the mean hemoglobin observed during AHI in a cohort of women in South Africa, the mean hemoglobin of women in our cohort was also higher both at baseline and after 48 weeks.15
The generalizability of our study may be limited by the predominantly Thai and male participants. Inherited hemoglobinopathy is common in parts of Thailand and was not tested routinely in our study.16 However, we encourage such testing in participants with anemia who may benefit from closer monitoring of hematologic parameters and possibly modifications of blood draw volume. This study is also limited by the use of hemoglobin as the sole assessment of anemia. Hence, the exact etiology of any case of anemia could not be fully evaluated. The local limit for blood donation volume was used for guidance in this cohort. It would be useful to have guidelines for blood volume parameters for clinical research in Thai adults that could be applicable to similar settings.
In this analysis, frequent blood draws in acutely HIV-infected participants did not lead to long-term declines in hemoglobin. Hemoglobin levels reverted back to normal during the first year for most of these participants who were on ART. Trial participants, researchers, and IRBs should be reassured of the safety of serial phlebotomy in this population and, if scientifically justified, might consider increasing the allowable volume of blood drawn for research purposes.
ACKNOWLEDGMENTS
The authors thank their study participants and staff from the Thai Red Cross AIDS Research Centre, Chulalongkorn University and AFRIMS for their valuable contributions to this study. The RV254/SEARCH 010 Study Group includes: from SEARCH/TRCARC/HIV-NAT: Praphan Phanuphak, Nipat Teeratakulpisarn, Mark de Souza, James Fletcher, Ponpen Tantivitayakul, Sasiwimol Ubolyam, Pacharin Eamyoung, Jintana Intasan, Duanghathai Sutthichom, P.P., Somprartthana Rattanamanee, S.P., Somporn Tipsuk, Khunthalee Benjapornpong, Nisakorn Ratnaratorn, Chutharat Munkong, Kamonkan, Tanjnareel; from AFRIMS: Robert J O'Connell, Siriwat Akapirat, Rapee Trichavaroj, Bessara Nuntapinit; from MHRP: Merlin Robb, Nelson Michael, Madelaine Ouellette, Oratai Butterworth.
Footnotes
Supported by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD) and by contributions from the Thai Red Cross AIDS Research Center. Antiretroviral therapy was supported by the Government Pharmaceutical Organization of Thailand Gilead, Merck and ViiV Healthcare.
The authors have no funding or conflicts of interest to disclose.
The views expressed are those of the authors and should not be construed to represent the positions of the participating institutions, the U.S. Army or the Department of Defense.
REFERENCES
- 1.WHO. Blood donor selection: guidelines on assessing donor suitability for blood donation. 2012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23700651\nhttp://www.who.int/bloodsafety/publications/BDSelection_WHOGuideAssessingDonorSuitability4BloodDonation.pdf?ua=1. Accessed 16 November 2016. [PubMed]
- 2.Wisser D. Blood loss from laboratory tests. Clin Chem. 2003;49:1651–1655. [DOI] [PubMed] [Google Scholar]
- 3.Stefanini M. Iatrogenic anemia (can it be prevented?). J Thromb Haemost. 2014;12:1591. [DOI] [PubMed] [Google Scholar]
- 4.Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volberding PA, Levine AM, Dieterrich D, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454–1463. [DOI] [PubMed] [Google Scholar]
- 6.Kinloch-de Loes S, Saussure P, Saurat J, et al. Symptomatic primary infection due to human immunodeficiency virus type 1: review of 31 cases. Clin Infect Dis. 1993;17:59–65. [DOI] [PubMed] [Google Scholar]
- 7.De Souza MS, Phanuphak N, Pinyakorn S, et al. Impact of nucleic acid testing relative to antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS. 2015;29:793–800. [DOI] [PubMed] [Google Scholar]
- 8.Phanuphak N, Teeratakulpisarn N, Van Griensven F, et al. Anogenital HIV RNA in Thai men who have sex with men in Bangkok during acute HIV infection and after randomization to standard vs. intensified antiretroviral regimens. J Int AIDS Soc. 2015;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Division of AIDS. National Institute of Allergy and Infectious Diseases. National Institutes of Health. Table for grading the severity of adult and pediatric adverse events. US Department of Health and Health Services; Bethesda, MD, USA; 2009. [Google Scholar]
- 10.Insiripong S, Yingsitsiri W, Boondumrongsagul J, et al. Prevalence of thalassemia traits in people without anemia or microcytosis. J Hematol Transfus Med. 2014;24:25–9. [Google Scholar]
- 11.Ines Egli TW. WHO Global Database on Anaemia. 2007. World Health Organization; http://who.int/vmnis/anaemia/data/database/countries/bgd_ida.pdf?ua=1. Accessed 16 November 2016. [Google Scholar]
- 12.Loscalzo J. Harrison's Principles of Internal Medicine. New York, NY: McGraw-Hill; 2012;2. [Google Scholar]
- 13.Renouf Shahvarani L, Sheps S, Hubley A, et al. The role of diet in predicting iron deficiency anemia in HIV-positive women. Can J Diet Pract Res. 2012;73:128–133. [DOI] [PubMed] [Google Scholar]
- 14.WHO. The Global Prevalence of Anaemia in 2011. World Health Organization, Geneva, Switzerland; 2015. [Google Scholar]
- 15.Mlisana K, Auld SC, Grobler A, et al. Anaemia in acute HIV-1 subtype C infection. PLoS One. 2008;3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yingsitsiri W, Boondumrongskul J. Thalassemia and hemoglobinopathy despite normal level of hemoglobin concentration and normal mean corpuscular volume. 2012;37:215–221. [Google Scholar]


