BACKGROUND:
After treatment with local excision for TNM stage I low rectal cancer, the risk of local recurrence is not only high for T2 lesions but also for T1 lesions with features of massive invasion to the submucosal layer and/or lymphovascular invasion.
OBJECTIVE:
The purpose of this study was to determine the efficacy of chemoradiotherapy combined with local excision in the treatment of T1 to T2 low rectal cancer.
DESIGN:
We conducted a prospective, single-arm, phase II trial.
SETTINGS:
This was a multicenter study.
PATIENTS:
From April 2003 to October 2010, 57 patients were treated with local excision after additional external beam irradiation (45 Gy) plus continuous 5-week intravenous injection of 5-fluorouracil (250 mg/m2 per day) at 10 domestic hospitals. Fifty-three patients had clinical T1N0 lesions, and 4 had T2N0 lesions in the low rectum, located below the peritoneal reflection.
MAIN OUTCOMES MEASURES:
The primary end point was disease-free survival at 5 years.
RESULTS:
The completion rate for full-dose chemoradiotherapy was 86% (49/57). Serious, nontransient treatment-related complications were not reported. With a median follow-up of 7.3 years after local excision, the 5-year disease-free survival rate was 94% for the 53 patients with T1 lesions and 75% for the 4 patients with T2 lesions. There were 2 local recurrences during the entire observation period. Anal function after local excision and chemoradiation were kept at almost the same levels as observed before treatment.
LIMITATIONS:
The study was limited by the small number of registered T2 rectal cancers, retrospective evaluations of quality of life, and the exclusion of poorly differentiated adenocarcinoma (a high-risk feature of T1 lesions).
CONCLUSIONS:
The addition of chemoradiotherapy to local excision of T1 rectal adenocarcinomas with poor prognostic features including deep submucosal invasion and lymphovascular invasion could improve on less favorable historic oncologic outcomes of local excision alone in this high-risk group for lymph node metastasis. See Video Abstract at http://links.lww.com/DCR/A421.
Keywords: Adjuvant chemoradiotherapy, Local excision, Rectal cancer
For cases of distal rectal cancer, local excision (LE) has been regarded as an attractive therapeutic method that is comparable with total mesorectal excision (TME). In the past, LE of rectal cancer was not considered to be a standard therapy because of high rates of local recurrence; instead, it was regarded as an option for relatively early stage rectal cancer, cases in which radical surgery was contraindicated, and patients who wanted to preserve their sphincter function. Local recurrences were reported to develop after LE for low to midrectal cancer in 0% to 20% of patients with T1 lesions and 0% to 37% of patients with T2 lesions.1–19 However, the improvements in LE have allowed patients to maintain quality of life (QoL) and have provided local control rates of 0% to 8% in T1 disease and 0% to 19% in T2 disease, as a result of combinations of LE with radiation or chemoradiation therapy (CRT) before or after conservation surgery or as a result of appropriately selected indications.20
According to the guidelines of the National Comprehensive Cancer Network, radical surgery and CRT are the adjuvant therapy options for rectal lesions that are diagnosed after transanal LE and are stage pT2 or pT1 with high-risk features. High-risk features include positive margin, lymphovascular invasion, poorly differentiated tumor, or invasion into the lower third of the submucosa (relative evaluation). On the other hand, Kitajima et al21 reported that there is growing evidence to support the theory that lesions having submucosal invasion limited to <1000 µm (absolute distance evaluation), without lymphovascular invasion and/or poorly differentiated components, do not result in lymph node metastasis (LNM). According to the Paris Classification and Japanese clinical guidelines, submucosal invasive colorectal cancer lesions are classified as being at high risk for LNM if they have any of the following features: submucosal invasion of more than 1000 µm, presence of lymphovascular invasion, and/or poorly differentiated components.22,23 The Japanese clinical guidelines also note that transabdominal resection, including TME, is the only recommended therapy for T1 lesions with high-risk features, T2 lesions, and margin-positive cases after LE.
We planned this multicenter clinical study to expand the new therapeutic options for early stage low rectal cancers. The objective of this study was to evaluate the short- and long-term outcomes of LE plus CRT as a therapeutic method that could be presented to patients as a treatment option for T1 and T2 rectal cancers with an LNM rate of 10% to 30%. In the present study, CRT (rather than the conventional standard radical resection) was administered to patients with T1 to T2 low rectal cancers that had been pathologically diagnosed as possessing high-risk factors for LNM after local transanal resection.
The choice between postoperative functional prognosis and radical cure depends on the preferences and requests of individual subjects. Therefore, we selected a single-arm design for this clinical study.
PATIENTS AND METHODS
Study Design and Approval
The study was a single arm, multicenter phase II trial. The study was registered as a clinical trial in the Japanese University Hospital Medical Information Network (registration No. 000011417). A central institutional review board and the institutional review board at each participating institution approved the study. All of the patients provided signed informed consent before enrollment.
Patients and Eligibility Criteria of the Study
The study cohort included patients who were treated at any of 10 hospitals between April 2003 and October 2010. All of the patients were required to have an Eastern Cooperative Oncology Group performance status of ≤1, be between the ages of 20 and 75 years, and have low rectal cancer located below the peritoneal reflection. In addition, biopsy examination had to show well- to moderately differentiated adenocarcinoma. The inclusion criteria for T1 lesions did not restrict the tumor size; instead, it was only necessary for the tumor to be resectable with a ≥1-cm horizontal margin by LE. In contrast, for T2 lesions, the greatest tumor diameter was required to be ≤3 cm. The clinical TNM stage was required to be T1N0 or T2N0, as established by all of the following: MRI, CT, proctoscopy, and digital rectal examination. The choice of whether to use endorectal ultrasound for diagnosing TNM stage was left to the policy of each institute.
After transanal full-thickness LE, the specimen was subjected to pathological examination to evaluate the risks of local or nodal recurrences. Patients who had pT3, margin-positive, or margin-unevaluable tumors were ineligible for the study. Similarly, patients with intramucosal carcinoma or carcinoma with slight submucosal invasion (<1000 µm) without any lymphovascular invasion were also excluded from the study, because they had a very low risk of recurrence. The inclusion criteria for CRT contained the following requirements: diagnosis with well- to moderately differentiated low rectal cancer, margin-negative T1 to T2 disease, and ≥1 high-risk feature for nodal metastasis for T1 lesion (based on pathological examination of an LE specimen). The high-risk features for T1 lesions included >1000-µm submucosal invasion and/or the presence of lymphovascular invasion.
Surgery
All of the LEs were performed under general, spinal, or local anesthesia. The patient’s position (prone jackknife or lithotomy position) and the method of anesthesia were decided by each surgeon. The study protocol did not include restrictions on the approach or method of the tumor resection (eg, endoscopic or nonendoscopic). A full-thickness excision of the tumor was performed with a 1-cm negative margin. Each surgeon decided whether the defect of the rectal wall should be repaired by suturing or should be left open.
Adjuvant CRT
Patients were reregistered when pathological findings showed eligibility, and CRT was started 3 to 8 weeks after LE. Radiotherapy was delivered with megavoltage equipment (>6 MV) using a 3- or 4-field technique. CT-based treatment planning was required. A total dose of 45 Gy was administered in 25 fractions of 1.8 Gy and was combined with continuous 5-week intravenous injection of 5-fluorouracil (250 mg/m2 per day). The clinical target volume (CTV) included the primary tumor bed and mesorectum. The border of the CTV was set as follows: the cranial border at 5 cm to the oral side from the primary tumor bed, the caudal border at 2 cm below the lowest tumor border, and the anterior border on axial sections containing the bladder, prostate, seminal vesicles, uterus, or vagina at 1 cm anterior from the mesorectal border, to account for the effect of bladder volume variation. The planning target volume was defined as the CTV plus a 0.5- to 1.0-cm margin, to account for internal organ motion and daily setup error. Because none of the patients had gross tumor after margin-negative LE, and because early staged rectal cancer rarely causes LNM to the pelvic sidewall, we selected the lower prophylactic radiation dose (45 Gy) only for the mesorectum, without tumor bed boost. For the same reasons, the CTV did not include the pelvic sidewall area. The National Cancer Institute Common Toxicity Criteria, version 2.0, was used to score the toxicity of CRT.
Comparison With Radical Surgery Cases
The current trial was not performed in a phase III setting and did not include comparisons with the outcomes of radical surgery or LE alone. Nonetheless, we contacted participating institutions and retrospectively collected data about radical surgeries that had been performed for T1 low rectal cancer during the same period. QoL data were also collected from eligible T1 patients for comparisons with the QoL of patients after radical surgeries. The postoperative anal function was summarized using the grading of Kirwan et al,24 based on data from 59 patients who received radical surgery and 50 patients from our T1 cohort (for whom sufficiently detailed data were available).
End Points and Statistical Analysis
Disease-free survival (DFS) at 5 years was selected as the primary end point for the current study. The secondary end points were 5-year overall survival (OS) and 5-year local recurrence-free survival (LRFS). In addition, adverse events (AEs) of LE and adjuvant CRT were recorded and aggregated over the entire observation period. Survival rates were estimated using the Kaplan–Meier method.
RESULTS
Patient and Tumor Characteristics
A total of 82 patients with low rectal cancer were enrolled from 10 domestic institutions. All of the patients received conventional transanal LE after registration. Based on their pathological examinations, 61 patients were regarded as candidates for adjuvant CRT, of whom 57 patients provided informed consent and were reregistered for CRT. A flow chart of all 82 patients is shown in Fig. 1, including a summary of their treatments. Twenty-one of the 82 patients were considered as postsurgically ineligible based on their pathological features: 2 patients had pT3 tumors and were recommended for radical surgery; 14 patients had pTis or slightly invasive (<1000 µm) submucosal cancer without risk factors for local or nodal recurrences and were followed-up observationally without any additional treatment; and 5 patients had positive or unevaluable margins and were also recommended for TME. No cases were excluded because of the presence of poorly differentiated components. Of the 61 patients who were eligible for this study, 4 did not agree to receive any adjuvant treatments and were excluded from the second registration. Of these 4 patients, 1 who had a T2 tumor developed local and distant recurrences and died of the original disease in 1.9 years after LE (Fig. 1). The remaining 3 patients had T1 lesions and achieved long survival times without any recurrence.
FIGURE 1.
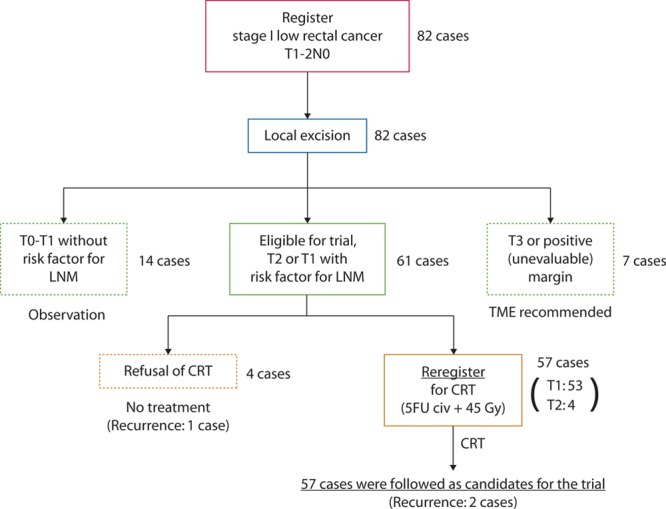
Trial protocol schema, including the enrolled patients, treatments, and outcomes. LNM = lymph node metastasis; CRT = chemoradiation therapy; 5FU = 5-fluorouracil; civ = continuous intravenous infusion.
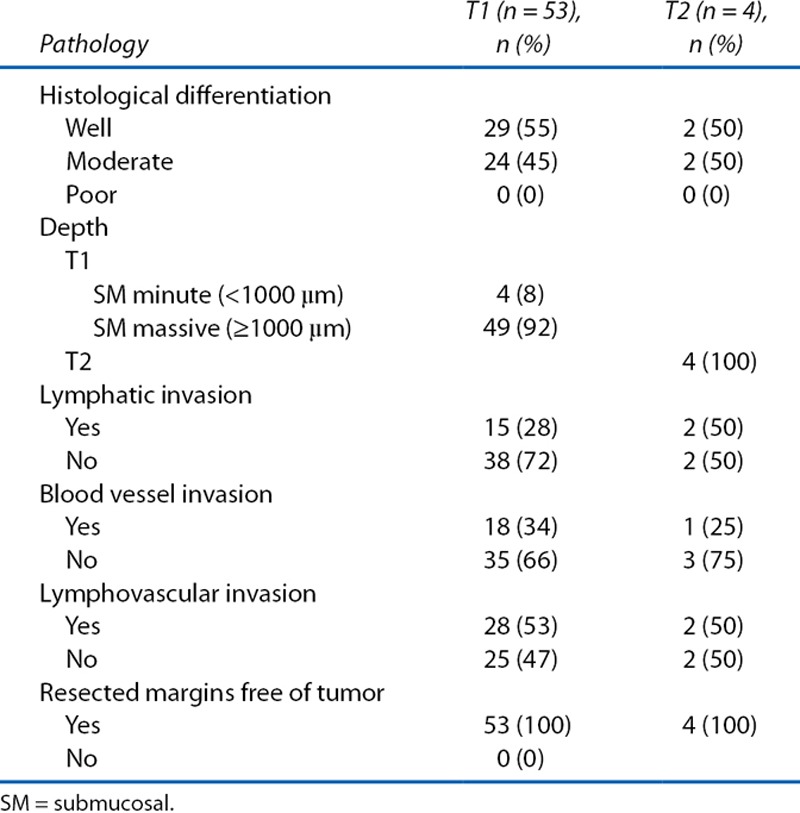
The patient and tumor characteristics of the 57 patients who were treated with LE plus adjuvant CRT are shown in Tables 1 and 2. Fifty three had T1 tumors with risk factors for local or nodal relapse, whereas 4 patients had T2 tumors. The ratio of the histological types of the tumors (well:moderate:poor differentiation) was 31:26:0. Regarding risk factors of nodal recurrence for T1 lesions, 28% and 34% of the tumors had lymphatic and vascular invasion, meaning that a combined 53% of the patients had vascular invasion, lymphatic invasion, or both.
TABLE 1.
Patient and disease characteristics
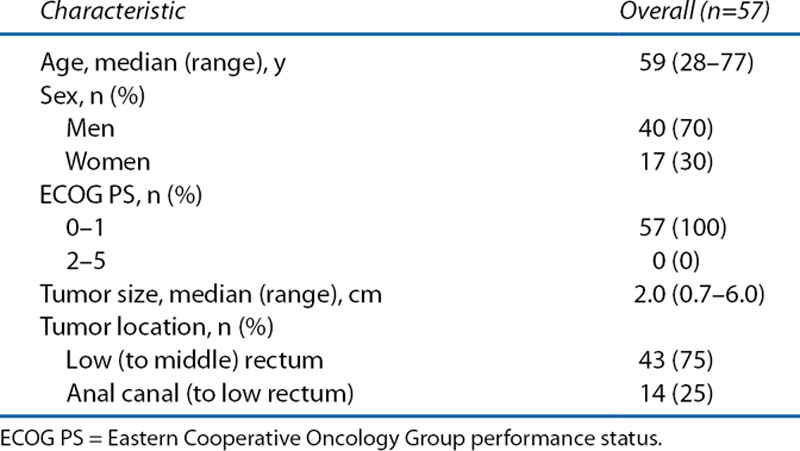
TABLE 2.
Pathological tumor characteristics
Surgery-Related Complications and CRT-Related AEs
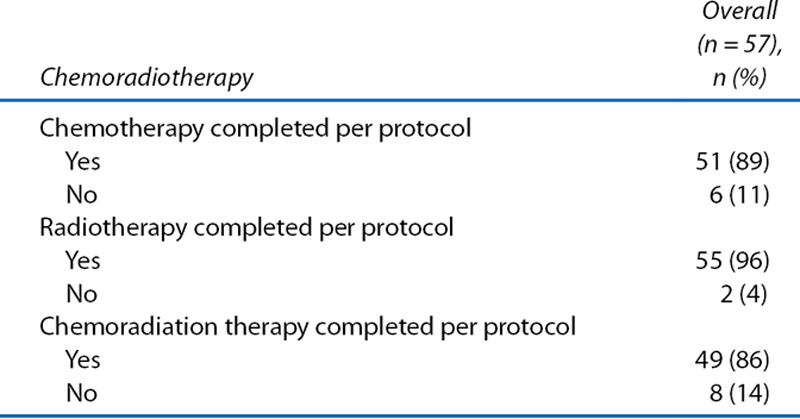
None of the 57 patients who received LE had recorded serious surgery-related complications, with the exception of 2 patients who developed fever of grade ≤2. On the other hand, overall, 20 patients developed 26 events of CRT-related AEs (Table 3). Two of these patients had 3 events of grade 3 AEs. The most common CRT-related AEs were diarrhea and anal pain. The treatment completion rate for full-dose CRT was 86% (49/57): 5 patients received dose-diminished chemotherapy, 2 patients discontinued chemotherapy, and 1 patient discontinued CRT because of AEs (Table 4). There was no report of any radiation-induced late effects during the observation period (Table 3).
TABLE 3.
Adverse events during chemoradiotherapy (N = 57)
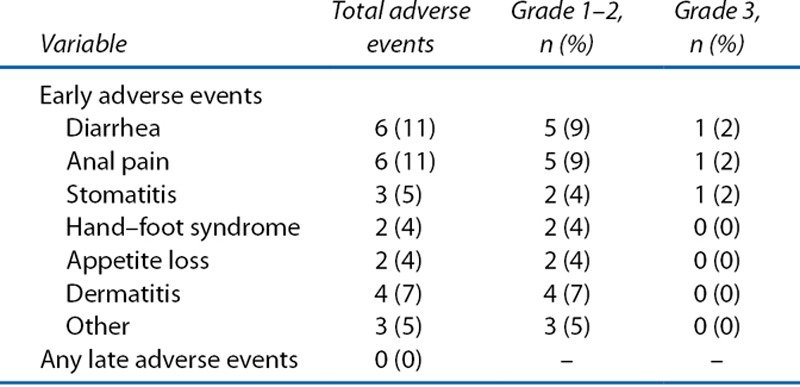
TABLE 4.
Chemoradiotherapy intervention
Oncologic Outcomes
With a median follow-up of 7.3 years after LE, the 5-year DFS was 94% (95% CI, 83%–98%) for the 53 patients with T1 lesions (Fig. 2). The 5-year LRFS for T1 cohort was 98% (95% CI, 87%–99%). The 5-year OS rate of the 53 patients with T1 lesions was 98% (95% CI, 86%–99%; Fig. 3). During the entire observation period, 2 patients developed local recurrences. One of these patients died of distant metastasis of rectal cancer, which occurred after radical salvage (survival time after LE, 90 mo), and the other died of other causes (survival time after LE, 100 mo). One patient who did not show evidence of recurrence also died of other causes (Table 5). Figure 4 presents the detailed characteristics, salvage surgery, and prognosis of the 2 patients who experienced relapse. Intersphincteric resection (ISR) and abdominoperineal resection (APR) were performed radically as salvage surgery.
FIGURE 2.
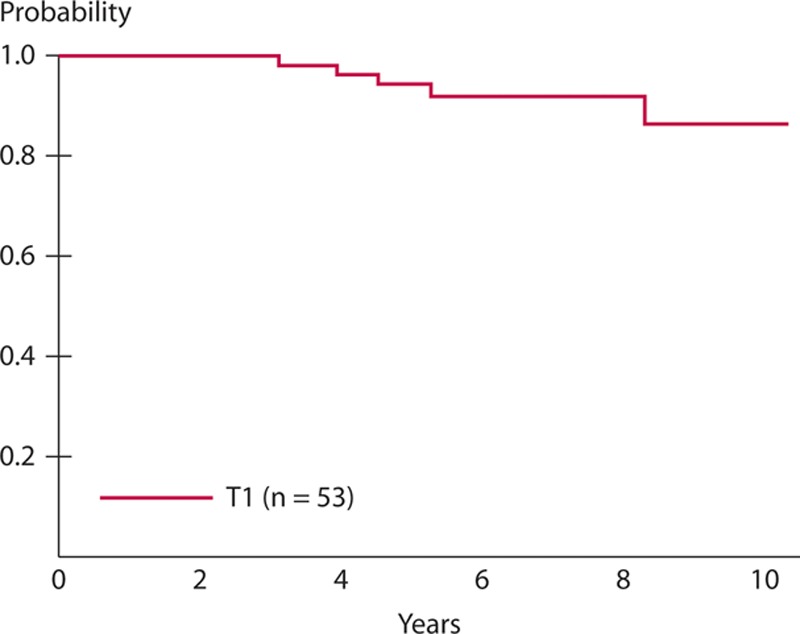
Disease-free survival of T1 cohort after a median follow-up of 7.3 y from local excision, the 5-y disease-free survival rate was 94% (95% CI, 83%–98%) for the 53 patients with T1 lesions.
FIGURE 3.
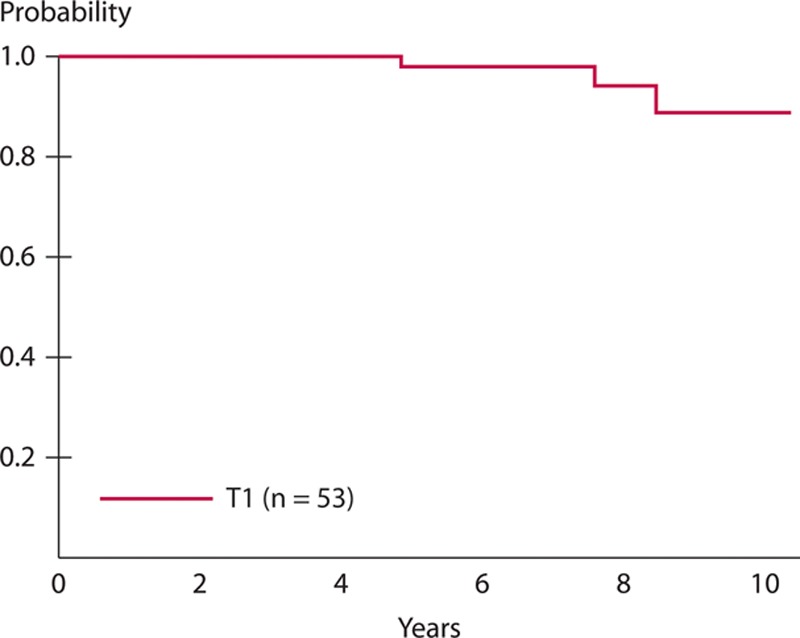
The 5-y overall survival rate of the 53 patients with T1 lesions was 98% (95% CI, 86%–99%).
TABLE 5.
Prognosis of patients with T1 and T2 lesions at 87 mo, the median duration of follow-up (range, 21–137 mo)
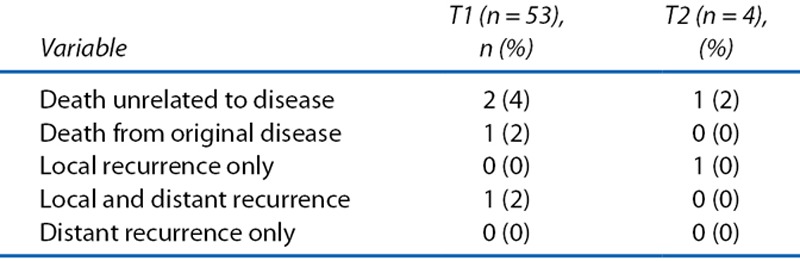
FIGURE 4.
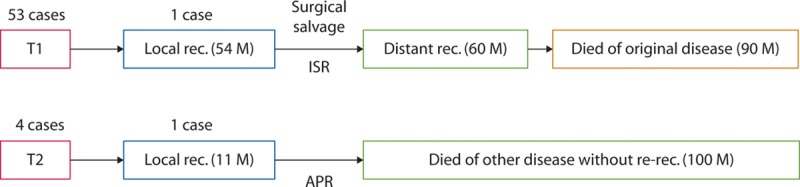
The courses of the relapsed patients. It was possible to perform surgical salvage in both of the patients who had local relapses and to control local disease successfully in both cases. APR = abdominoperineal resection; ISR = intersphincteric resection; M: months; rec. = recurrence.
Comparison With Radical Surgery Cases
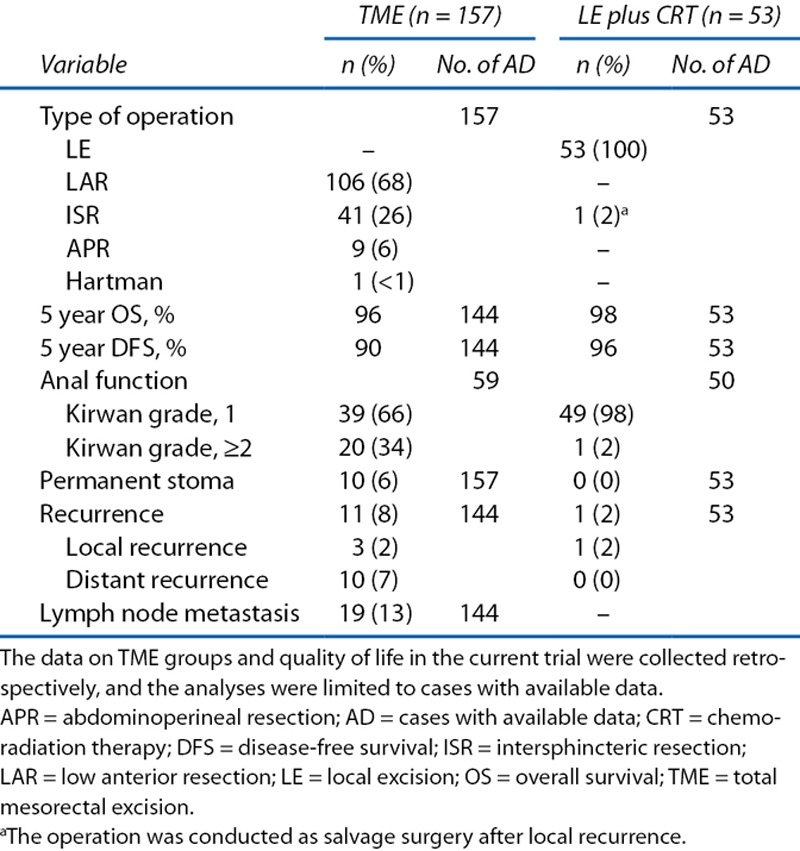
At 5 institutions that registered 50 (94%) of 53 patients in our T1 eligible cohort, a total of 157 cases of radical surgery for T1 low rectal cancer were performed (Table 6). This indicates that we were able to recruit approximately one third of the patients who were thought to be suitable for our study. The 5-year DFS and OS rates for patients who received these radical surgeries were 90% and 96% (n = 144). In addition, we observed that local recurrence and distant recurrence were developed by 2% and 7% of patients who received radical surgeries (n = 144). Regarding the QoL of patients who received radical surgeries, 34% had a Kirwan grade of ≥2 (n = 59), and 6% possessed permanent stoma (n = 157). In contrast, only 1 (2%) of the 50 patients in our T1 cohort, which did not include patients who underwent salvage surgery, had a Kirwan grade of ≥2, indicating that most of the patients did not have any stool incontinence issues.
TABLE 6.
Comparison of treatment outcomes of T1 low rectal cancer between TME and LE plus CRT
DISCUSSION
In the current study, LE and adjuvant CRT for T1 low rectal cancer provided acceptable oncologic outcomes. The 5-year DFS and LRFS outcomes were 94% and 98% in the T1 cohort. Two local recurrences were noted, and salvage resections (ISR and APR) were carried out with a 50% rate of rerelapse. The full dose of CRT was completed by 86% of all patients without serious, nontransient complications. The target and plan of irradiation that we set did not include the pelvic sidewall area or tumor bed boost, which might have reduced the AEs associated with the radiation therapy. Salvage surgery after CRT was also performed safely in 2 patients, without serious complications.
On the other hand, we were only able to recruit 4 patients who had T2 lesions, which prevented us from evaluating or discussing the results for the T2 cohort. In series of T2 cases treated with LE plus adjuvant CRT, local relapse rates have been as high as 18.0%25 and 21.8%.26 In contrast, the 2010 Japanese guidelines for the treatment of colorectal cancer note that the recurrence rate of T2 rectal cancer after radical resection was 8.6% (including distal metastasis).23 This appears to be the main reason that few patients with T2 disease were included in the present study, indicating that Japanese surgeons were hesitant to select a treatment other than TME for T2 low rectal lesions.
In a global trend, LE and LE plus CRT have become widely accepted and reported as therapeutic options for early stage rectal cancer. Naturally, LE for rectal cancer is superior to radical surgery in that it does not have such negative effects on QoL, including those resulting from permanent colostomy or long-term anal, bowel, urinary, or sexual dysfunction. Fifty five of the 57 patients in our study successfully avoided radical resections, such as APR and ISR, as well as very low anterior resection.26 Although specific data were not collected prospectively on anal, urinary, or sexual function after LE plus CRT, there was no report that LE plus CRT had affected a patient’s QoL to the same degree as radical surgery. QoL was also analyzed retrospectively from a subset of the cooperating institutions that participated in the present study. The results indicate that LE plus CRT is clearly superior to TME in terms of maintaining patients’ anal function. Pucciarelli et al27 showed that patients who underwent CRT followed by LE had better QoL and bowel function than those who underwent CRT followed by mesorectal excision. Their study used the QoL questionnaire-specific colorectal module of the European Organization for Research and Treatment of Cancer and the Memorial Sloan–Kettering Cancer Center Bowel Function Instrument. Especially for stool frequency, impotence, incomplete emptying after a bowel movement, and stool fractionation, scores were better in the LE group than in the conventional TME group, and the differences were statistically significant.
In a study of the long-term outcomes after radical resection for T1 rectal cancers with high risks of LNM, local recurrence was only observed in 0.6% (1/156) of cases, and the 5-year DFS rate was 95%.28 The same study also investigated the natural course of high-risk T1 disease in patients who did not receive any additional treatment after local submucosal excision. Local recurrence was seen in 14% (5/37) of these patients, and their 5-year DFS rate was 78%, although resection margins were negative. In an analysis of patients with T1 rectal cancer, Nash et al29 also reported that the local recurrence rate after radical resection was lower than that after transanal excision without subsequent treatment (the local recurrence rate was 14% in the LE group and 3% in the radical resection group). It has been demonstrated that CRT after LE significantly reduces the recurrence of high-risk T1 lesions. Min et al30 showed that, even for pT1 lesions, LE alone fails to demonstrate an acceptable oncologic outcome. They reported that patients with T1 rectal cancer who received adjuvant radiation therapy after LE had a 5-year LRFS rate of 100%, whereas those who did not receive adjuvant radiation therapy had a 5-year LRFS of 83.8%.
Modern imaging is becoming more important for the accurate diagnosis of clinical stage and the appropriate selection of patients for LE. One of the most reliable diagnostic modalities for detecting T1 lesions is magnifying chromoendoscopy, which can effectively detect invasive or noninvasive patterns and estimate the depth of invasion of early rectal cancers that are classified as having mucosal crypt orifices (pit pattern).31 The 2 other imaging modalities that are used to diagnose clinical TNM stage are endorectal ultrasound and MRI.32–35 Digital rectal examination can provide valuable information, such as the tumor location, distance from the anal verge, size, and mobility. However, it also depends on the surgeon’s level of experience. There are several remaining obstacles regarding the choice of the best surgical approaches and adjuvant therapies for T1 and T2 low rectal cancer, especially when considering the risks of local recurrences and functional issues: the need for accurate diagnosis in clinical TNM staging.
CONCLUSION
The addition of chemoradiotherapy to LE of T1 rectal adenocarcinomas with poor prognostic features including deep submucosal invasion and lymphovascular invasion could improve on less favorable historic oncologic outcomes of LE alone in this high-risk group for LNM. The addition of chemoradiotherapy to LE was not associated with major changes in QoL or the risks of severe treatment-related AEs.
ACKNOWLEDGMENTS
The authors thank all of the doctors (surgeons, medical oncologists, and radiologists) from the 10 institutes that participated in this study, especially Toshihiko Sato (Department of Surgery, Yamagata Prefectural Central Hospital) and Kazuhito Minami (Department of Gastroenterological Surgery, National Kyushu Cancer Center). The present study was planned by Team Moriya in, “A Study with the Objective of Standardizing the Diagnosis, Therapies, and Follow-up in Bowel Cancer,” for Team Ebihara’s, “Collaborative Study on the Standardization of Cancer Therapy Utilizing Special Cancer Medical Facilities,” under the grant-in-aid for cancer research designation of the Ministry of Health and Welfare of Japan.
Footnotes
Funding/Support: This work was supported by a grant-in-aid for cancer research designation by the Ministry of Health and Welfare of Japan.
Financial Disclosure: None reported.
REFERENCES
- 1.Hager T, Gall FP, Hermanek P.Local excision of cancer of the rectum. Dis Colon Rectum. 1983;26:149–151. [DOI] [PubMed] [Google Scholar]
- 2.Stearns MW, Jr, Sternberg SS, DeCosse JJ.Treatment alternatives: localized rectal cancer. Cancer. 1984;54(11 suppl):2691–2694. [DOI] [PubMed] [Google Scholar]
- 3.Whiteway J, Nicholls RJ, Morson BC.The role of surgical local excision in the treatment of rectal cancer. Br J Surg. 1985;72:694–697. [DOI] [PubMed] [Google Scholar]
- 4.Biggers OR, Beart RW, Jr, Ilstrup DM.Local excision of rectal cancer. Dis Colon Rectum. 1986;29:374–377. [DOI] [PubMed] [Google Scholar]
- 5.Billingham RP.Conservative treatment of rectal cancer: extending the indications. Cancer. 1992;70(5 suppl):1355–1363. [DOI] [PubMed] [Google Scholar]
- 6.Coco C, Magistrelli P, Granone P, Roncolini G, Picciocchi A.Conservative surgery for early cancer of the distal rectum. Dis Colon Rectum. 1992;35:131–136. [DOI] [PubMed] [Google Scholar]
- 7.Heimann TM, Oh C, Steinhagen RM, Greenstein AJ, Perez C, Aufses AH., JrSurgical treatment of tumors of the distal rectum with sphincter preservation. Ann Surg. 1992;216:432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham RA, Atkins MB, Karp DD, Wazer DE, Hackford AW.Local excision of rectal carcinoma: early results with combined chemoradiation therapy using 5-fluorouracil and leucovorin. Dis Colon Rectum. 1994;37:308–312. [DOI] [PubMed] [Google Scholar]
- 9.Frazee RC, Patel R, Belew M, Roberts JW, Hendricks JC.Transanal excision of rectal carcinoma. Am Surg. 1995;61:714–717. [PubMed] [Google Scholar]
- 10.Bleday R.Local excision of rectal cancer. World J Surg. 1997;21:706–714. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RH, Hay JH, Larsson SN.Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175:360–363. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham RA, Hackford AW, Wazer DE.Local excision of rectal carcinoma: a safe alternative for more advanced tumors? J Surg Oncol. 1999;70:235–238. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki Y, Takeda Y, Miyahara T, Tokutome T.Evaluation of local excision for sessile-type lower rectal tumors. Hepatogastroenterology. 1999;46:2329–2332. [PubMed] [Google Scholar]
- 15.Steele GD, Jr, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol. 1999;6:433–441. [DOI] [PubMed] [Google Scholar]
- 16.Varma MG, Rogers SJ, Schrock TR, Welton ML.Local excision of rectal carcinoma. Arch Surg. 1999;134:863–867. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA.Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J.Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071. [DOI] [PubMed] [Google Scholar]
- 19.Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Radiat Oncol Biol Phys. 2000;46:313–322. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta S, Tjandra JJ.Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44:1345–1361. [DOI] [PubMed] [Google Scholar]
- 21.Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. [DOI] [PubMed] [Google Scholar]
- 22.Inoue H, Kashida H, Kudo S, et al. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. [DOI] [PubMed] [Google Scholar]
- 24.Kirwan WO, Drumm J, Hogan JM, Keohane C.Determining safe margin of resection in low anterior resection for rectal cancer. Br J Surg. 1988;75:720. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg JA, Shibata D, Herndon JE, 2nd, Steele GD, Jr, Mayer R, Bleday R.Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum. 2008;51:1185–1191. [DOI] [PubMed] [Google Scholar]
- 26.Rackley TP, Ma RM, Brown CJ, Hay JH.Transanal local excision for patients with rectal cancer: can radiation compensate for what is perceived as a nondefinitive surgical approach? Dis Colon Rectum. 2016;59:173–178. [DOI] [PubMed] [Google Scholar]
- 27.Pucciarelli S, Giandomenico F, De Paoli A, et al. Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg. 2017;104:138–147. [DOI] [PubMed] [Google Scholar]
- 28.Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–9; quiz e14. [DOI] [PubMed] [Google Scholar]
- 29.Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52:577–582. [DOI] [PubMed] [Google Scholar]
- 30.Min BS, Kim NK, Ko YT, et al. Long-term oncologic results of patients with distal rectal cancer treated by local excision with or without adjuvant treatment. Int J Colorectal Dis. 2007;22:1325–1330. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700–2706. [DOI] [PubMed] [Google Scholar]
- 32.Hassan I, Wise PE, Margolin DA, Fleshman JW.The role of transanal surgery in the management of T1 rectal cancers. J Gastrointest Surg. 2015;19:1704–1712. [DOI] [PubMed] [Google Scholar]
- 33.Bellows CF, Jaffe B, Bacigalupo L, Pucciarelli S, Gagliardi G.Clinical significance of magnetic resonance imaging findings in rectal cancer. World J Radiol. 2011;3:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beets-Tan RG, Beets GL.Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335–346. [DOI] [PubMed] [Google Scholar]
- 35.Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR.Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255–1265. [DOI] [PubMed] [Google Scholar]


