Abstract
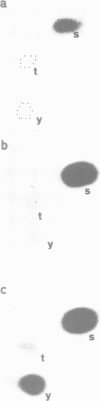
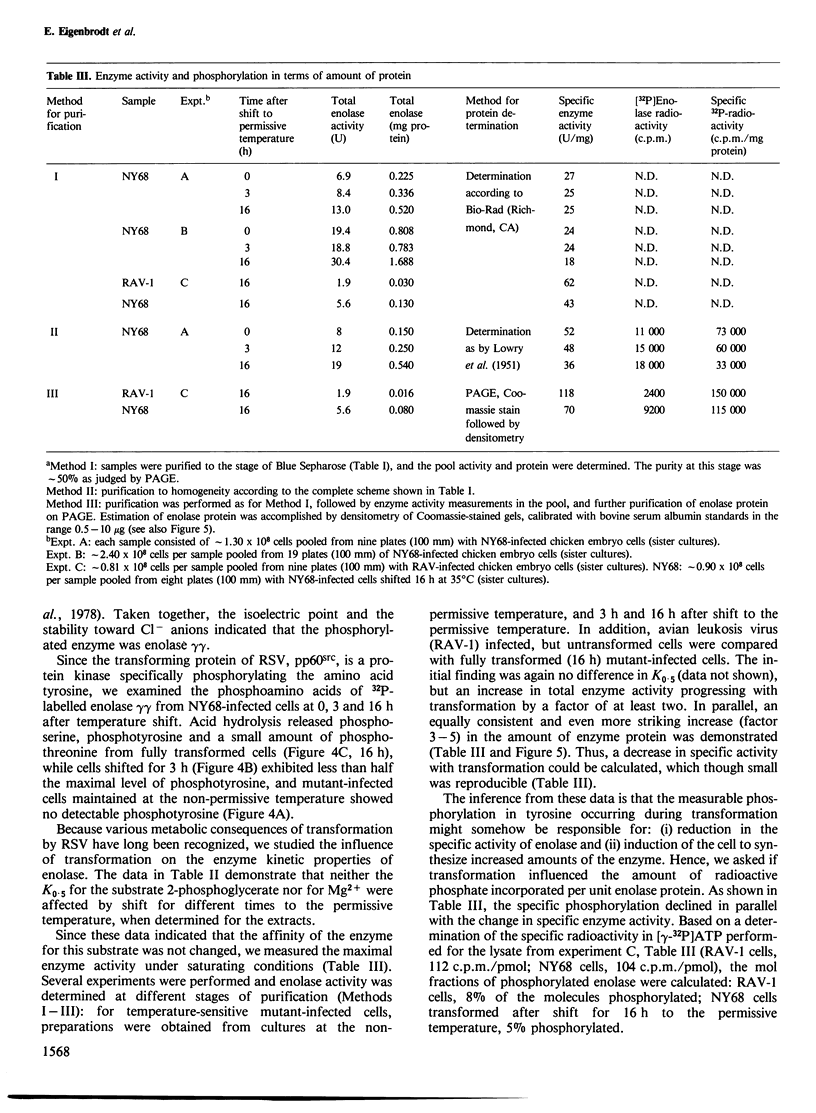
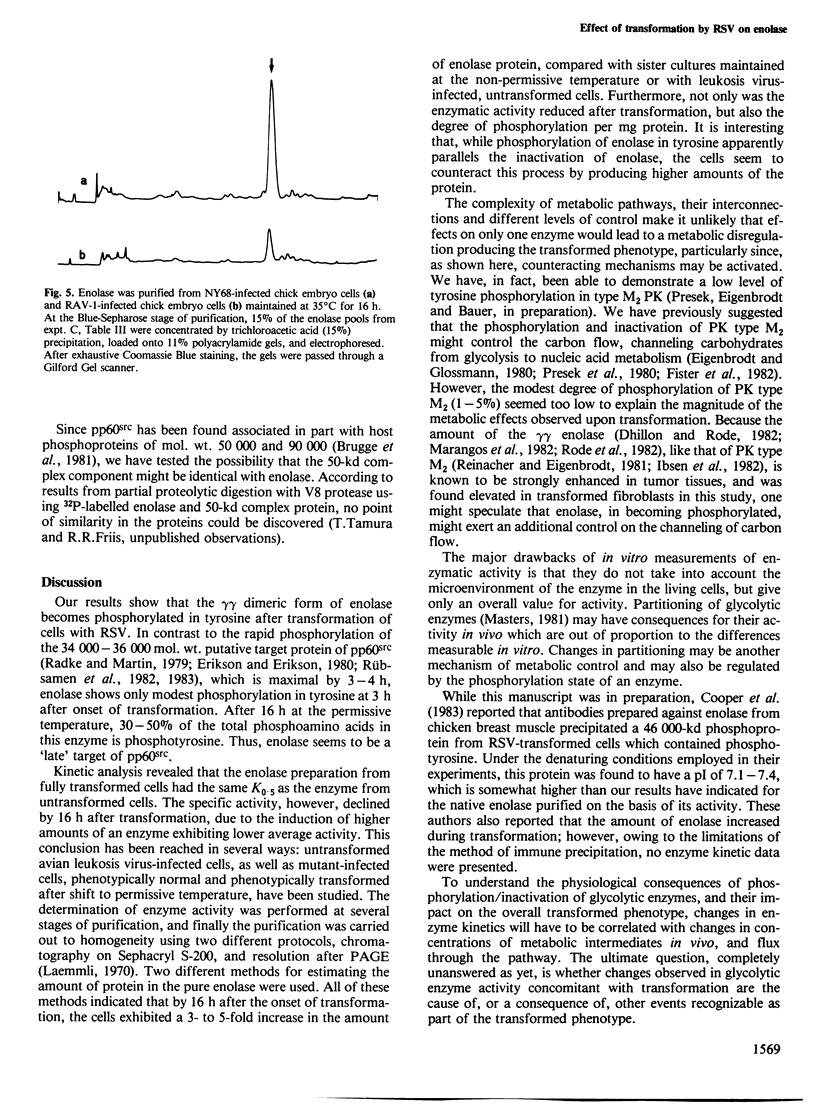
Using chicken embryo fibroblasts infected with the NY68 transformation-defective temperature-sensitive mutant of Rous sarcoma virus, the phosphorylation and enzyme kinetic properties of enolase have been studied before, and at different stages after, the onset of transformation. A method for purification of enolase was developed, which minimized dephosphorylation. Two enolase (EC 4.2.1.11) isoenzymes were separated by isoelectric focussing revealing that it was the gammagamma form (pI 5.2-6.7) which had become phosphorylated at tyrosine residues after transformation. The phosphorylation of enolase in tyrosine occurred slowly after shift to the permissive temperature, rising from undetectable levels in phenotypically normal cells, to < 10% of the total phosphoamino acid after 3 h, and reaching 30-50% of the total phosphoamino acid by 16 h. Interestingly, the fraction of phosphorylated enolase molecules declined during transformation from 8% in normal cells to 5% by 16 h after temperature shift, due to a 3- to 5-fold increase in the total amount of enolase present in the transformed cultures. Although transformation had no apparent effect on the K0.5 of enolase (26 +/- 4 microM for 2-phosphoglycerate), its specific activity was reduced by about one third.
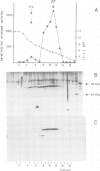
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Burk D., Woods M., Hunter J. On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J Natl Cancer Inst. 1967 Jun;38(6):839–863. [PubMed] [Google Scholar]
- Carroll R. C., Ash J. F., Vogt P. K., Singer S. J. Reversion of transformed glycolysis to normal by inhibition of protein synthesis in rat kidney cells infected with temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5015–5019. doi: 10.1073/pnas.75.10.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Dhillon A. P., Rode J. Patterns of staining for neurone specific enolase in benign and malignant melanocytic lesions of the skin. Diagn Histopathol. 1982 Jul-Sep;5(3):169–174. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Fister P., Eigenbrodt E., Schoner W. Simultaneous stimulation of uric acid synthesis and gluconeogenesis in chicken hepatocytes by alpha-adrenergic action of epinephrine and calcium. FEBS Lett. 1982 Mar 8;139(1):27–31. doi: 10.1016/0014-5793(82)80479-1. [DOI] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Stimulation of lactic acid production in chick embryo fibroblasts by serum and high pH in the absence of external glucose. J Cell Physiol. 1975 Dec;86(3 Pt 1):453–457. doi: 10.1002/jcp.1040860303. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Temperature-sensitive mutants of avian RNA tumor viruses: a review. Curr Top Microbiol Immunol. 1978;79:261–293. doi: 10.1007/978-3-642-66853-1_6. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Presek P., Eigenbrodt E. Association of the src-gene product of Rous sarcoma virus with a pyruvate-kinase inactivation factor. Mol Cell Endocrinol. 1981 Jul;23(1):49–63. doi: 10.1016/0303-7207(81)90116-7. [DOI] [PubMed] [Google Scholar]
- Gockel S. F., Lebherz H. G. "Conformational" isoenzymes of ascarid enolase. J Biol Chem. 1981 Apr 25;256(8):3877–3883. [PubMed] [Google Scholar]
- Hume D. A., Radik J. L., Ferber E., Weidemann M. J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978 Sep 15;174(3):703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibsen K. H., Orlando R. A., Garratt K. N., Hernandez A. M., Giorlando S., Nungaray G. Expression of multimolecular forms of pyruvate kinase in normal, benign, and malignant human breast tissue. Cancer Res. 1982 Mar;42(3):888–892. [PubMed] [Google Scholar]
- Johnson L. V., Summerhayes I. C., Chen L. B. Decreased uptake and retention of rhodamine 123 by mitochondria in feline sarcoma virus-transformed mink cells. Cell. 1982 Jan;28(1):7–14. doi: 10.1016/0092-8674(82)90369-5. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Campbell I. C., Schmechel D. E., Murphy D. L., Goodwin F. K. Blood platelets contain a neuron-specific enolase subunit. J Neurochem. 1980 May;34(5):1254–1258. doi: 10.1111/j.1471-4159.1980.tb09967.x. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Gazdar A. F., Carney D. N. Neuron specific enolase in human small cell carcinoma cultures. Cancer Lett. 1982 Jan;15(1):67–71. doi: 10.1016/0304-3835(82)90077-5. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Parma A. M., Goodwin F. K. Functional properties of neuronal and glial isoenzymes of brain enolase. J Neurochem. 1978 Sep;31(3):727–732. doi: 10.1111/j.1471-4159.1978.tb07847.x. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Zomzely-Neurath C., York C. Determination and characterization of neuron specific protein (NSP) associated enolase activity. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1309–1316. doi: 10.1016/0006-291x(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Masters C. J. Interactions between soluble enzymes and subcellular structure. CRC Crit Rev Biochem. 1981;11(2):105–143. doi: 10.3109/10409238109108700. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A. Changes in NAD(P)+-dependent malic enzyme and malate dehydrogenase activities during fibroblast proliferation. J Cell Physiol. 1982 Feb;110(2):142–148. doi: 10.1002/jcp.1041100206. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A. Oxocarboxylic acids, pyridine nucleotide-linked oxidoreductases and serum factors in regulation of cell proliferation. J Cell Physiol. 1979 Oct;101(1):9–16. doi: 10.1002/jcp.1041010103. [DOI] [PubMed] [Google Scholar]
- Nakano E. T., Ciampi N. A., Young D. V. The identification of a serum viability factor for SV3T3 cells as biotin and its possible relationship to the maintenance of Krebs cycle activity. Arch Biochem Biophys. 1982 May;215(2):556–563. doi: 10.1016/0003-9861(82)90116-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Presek P., Glossmann H., Eigenbrodt E., Schoner W., Rübsamen H., Friis R. R., Bauer H. Similarities between a phosphoprotein (pp60src)-associated protein kinase of Rous sarcoma virus and a cyclic adenosine 3':5'-monophosphate-independent protein kinase that phosphorylates pyruvate kinase type M2. Cancer Res. 1980 May;40(5):1733–1741. [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambeck W. A., Bissell M. J., Bassham J. A. Metabolism in normal and virus-transformed chick embryo fibroblasts as observed with glucose labeled with 14C and tritium and with tritium-labeled water. Hoppe Seylers Z Physiol Chem. 1975 Feb;356(2):203–212. doi: 10.1515/bchm2.1975.356.1.203. [DOI] [PubMed] [Google Scholar]
- Reinacher M., Eigenbrodt E. Immunohistological demonstration of the same type of pyruvate kinase isoenzyme (M2-Pk) in tumors of chicken and rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(1):79–88. doi: 10.1007/BF02892557. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Rode J., Dhillon A. P., Papadaki L. Immunohistochemical staining of granular cell tumour for neurone specific enolase: evidence in support of a neural origin. Diagn Histopathol. 1982 Jul-Sep;5(3):205–211. [PubMed] [Google Scholar]
- Rübsamen H., Saltenberger K., Friis R. R., Eigenbrodt E. Cytosolic malic dehydrogenase activity is associated with a putative substrate for the transforming gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):228–232. doi: 10.1073/pnas.79.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens D. A., Hochstadt B., Amos H. Effects of pyruvate on the growth of normal and transformed hamster embryo fibroblasts. J Cell Physiol. 1982 Mar;110(3):329–335. doi: 10.1002/jcp.1041100319. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. 8. Glycolysis and cell multiplication. Int J Cancer. 1968 Mar 15;3(2):273–282. doi: 10.1002/ijc.2910030213. [DOI] [PubMed] [Google Scholar]