Abstract
Background:
In this study, we evaluated assessed elements connected with low birth weight (LBW) and used decision curve analysis (DCA) to define a scale to anticipate the probability of having a LBW newborn child.
Methods:
This hospital-based case–control study was led in Arak Hospital in Iran. The study included 470 mothers with LBW neonate and 470 mothers with natural neonates. Information were gathered by meeting moms utilizing preplanned organized questionnaire and from hospital records. The estimated probabilities of detecting LBW were calculated using the logistic regression and DCA to quantify the clinical consequences and its validation.
Results:
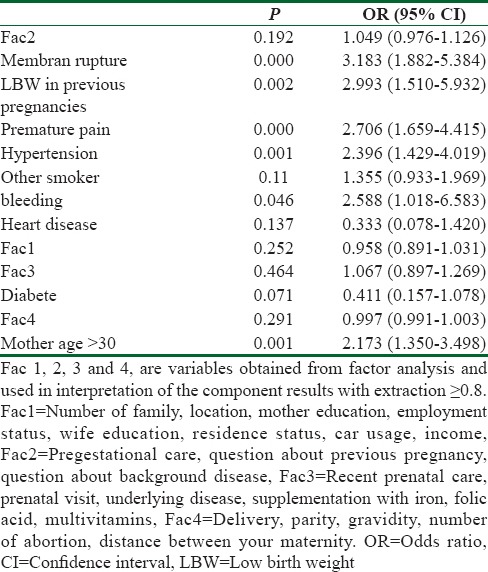
Factors significantly associated with LBW were premature membrane rupture (odds ratio [OR] = 3.18 [1.882–5.384]), former LBW infants (OR = 2.99 [1.510–5.932]), premature pain (OR = 2.70 [1.659–4.415]), hypertension in pregnancy (OR = 2.39 [1.429–4.019]), last trimester of pregnancy bleeding (OR = 2.58 [1.018–6.583]), mother age >30 (OR = 2.17 [1.350–3.498]). However, with DCA, the prediction model made on these 15 variables has a net benefit (NB) of 0.3110 is best predictive with the highest NB. NB has simple clinical interpretation and utilizing the model is what might as well be called a procedure that distinguished what might as well be called 31.1 LBW per 100 cases with no superfluous recognize.
Conclusions:
It is conceivable to foresee LBW utilizing a prediction model show in light of noteworthy hazard components connected with LBW. The majority of the hazard elements for LBW are preventable, and moms can be alluded amid early pregnancy to a middle which is furnished with facilities for administration of high hazard pregnancy and LBW infant.
Keywords: Decision curve analysis, low birth weight, maternal status, validation
Introduction
Low birth weight (LBW) is one of the fundamental hazard elements for newborn child morbidity and mortality.[1] As per World Health Organization (WHO), an infant weighing under 2500 g (5.5 pounds) during childbirth is named as LBW neonate.[2]
LBW is a multifactorial wonder. Numerous maternal and fetal variables are observed fundamentally to be connected with the LBW.[3] Although many risk factors associated with LBW have been distinguished, around half of all cases are of obscure ethology.[1]
LBW is connected with ahead of schedule and late bleak conditions, for example, coronary illness, noninsulin subordinate diabetes, adolescence hypertension, behavioral clutters, debilitated intellectual capacity, mental scatters, and these as a rule have long haul money-related weight[4] what's more, the event of hypertension, insulin resistance, hypercholesterolemia and hyperuricemia in grown-up life and the occurrence of hypertension, insulin resistance, and hyperuricemia in adult life.[5]
LBW and prematurity are the second driving reason for newborn child mortality after congenital anomalies but contribute disproportionately to the infant mortality rate (deaths in the 1st year after birth).[6] Infants with an LBW are 40 times more likely to die than newborn children with normal birth weight (NBW). Newborn children with LBW are at a much higher danger of being conceived with cerebral paralysis, mental hindrance, and other tangible and intellectual disabilities, contrasted with infants of NBW.[7]
Prediction models are utilized to assess the probability of the presence a specific disease (diagnosis) or to assess the probability of developing a specific result in the future (prognosis). New method based on decision curve analysis (DCA) has recently been introduce.[8]
DCA is a novel technique for assessing diagnostic tests, prediction models, and molecular markers. The key idea for this type of assessment is the “net benefit.”[9,10]
DCA joins the mathematical simplicity of accuracy measures, such as sensitivity and specificity, with the clinical applicability of decision analytic approaches. Most critically, DCA can be applied straightforwardly to a data set[10] and could identify the range of threshold probabilities, in which a model was of value, the magnitude of advantage, and which of several models was ideal.[9]
Diagnostic and prognostic models are ordinarily assessed with measures of accuracy that do not address clinical outcomes. Decision-analytic techniques permit assessment of clinical outcomes, but frequently require accumulation of extra data (costs, benefits, and preferences), and may be bulky to apply to models that yield a persistent outcome.[9]
However, in recent years, many researchers on the risk factors influencing the LBW have been working; however, for the first time, we looked a technique for assessing and contrasting prediction models that incorporates clinical outcomes, requires just the dataset on which the models are tested, and can be applied to models that have either continuous or dichotomous outcomes and the requirement for such a scale was strongly felt, and this study was attempted with the objective of ensuing a prediction scale for LBW.
Methods
This hospital-based case–control study was conducted in Arak Hospital in Iran. The sample size was calculated based on the LBW prevalence of 9.1%.[11]
Totally, 470 mothers of LBW infants (2500 g or less) referred to one of the hospitals in Arak town for delivery in 2014 were chosen as case group, and 470 mothers with NBW infants (weighing 2500–4000 g) participated in this study from that hospital as control group. In this study, the data were collected using pretested interviewer guided semi-structured checklist. The instrument was prepared reviewing similar literature[12] and was reviewed and completed by Arak Health Department.
The checklist consisting of ten parts (demographic specification, socioeconomic status, preconception cares, prenatal care, factors associated with childbirth, maternal exposure to tobacco products [passive smoker], medical history [specific drug consumption, history of heart disease, and diabetes], previous high-risk pregnancy [false labor, recurrent or late miscarriage, infants weighing <2500 g], complications of pregnancy and childbirth in the recent pregnancy [hypertension, premature membrane rupture, last trimester bleeding, false labor, multiple pregnancy, and gestational diabetes], and medical record reviews of newborns) and 72 items in pregnant women consider that these same papers more was evaluated according to the number of variable items that were selected. The main part of the data was collected through face-to-face maternal interview and other parts with medical record reviews of newborns. In questionnaire survey, talk on ethical issues and all participants completed the questionnaires voluntarily and with consent.
Statistical analysis
In this study, using comparisons between groups were performed using the Chi-square test with Yates correction (or Fisher's exact test and likelihood ratio where appropriate) for nominal variables, and the Student's t-test for continuous variables, and then, exploratory factor analysis of four variables, socioeconomic status, preconception cares, pregnancy cares, and pregnancy-related factors were made with principle component analysis method to reduce the data for subsequent analysis.
Second, aimed at fitting, logistic regression model on data obtained from the questionnaire to describe the relationship between the response variable (dependent) and a set of predictor variables (independent) in order of importance and the impact of each predictor variable on LBW infants, and selecting the best model with the highest NB using DCA. Accordingly, the most influential variables in predicting a LBW infant are determined.
This analysis is designed to calculate the clinical utility of prediction models. Using DCA, the relative impact of false-positive and false-negative results produced by the prediction model is measured to yield the “net benefit” for the model.[13]
The formula for NB goes back to work published long ago (first attributed to Peirce):[14]
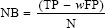
Where
TP: True positive count,
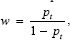
FP: False-positive count,
N: Total number of patients.
In this method, we utilize the hypothetical relationship between the threshold probability of disease and the relative value of false-positive and false-negative outcomes to determine the value of a prediction model.[9] To judge, whether pi is high sufficiently, one should weigh the profit P acquired by diagnosed an individual with the problem and the loss L caused by diagnosed an individual without the problem. Threshold probability defined by pt= L/(L + P). The threshold probability pt, and hence, the decision to selector not to select for the diagnosed, is thus a one-to-one function of the ratio L/P which is educational of how a clinician or a patient weighs the relative harms of false-positive and false-negative outcomes.[15]
When displayed graphically, the resulting curves illustrate the NB across all possible threshold probabilities (0–1) through weighing the relative harm of a false-positive or false-negative result to the benefit of a true-positive or true-negative result. As an additional assessment of clinical utility, the DCA curves of each model were also compared to two other theoretical scenarios: One, in which every cases be have LBW (all cases are correctly predicted and as sensitivity is 100% and specificity 0%) and one, in which no cases have LBW (zero), regardless of the probability of LBW. Description of the decision curve relies on comparing the NB of the test, model or marker with that of a procedure of “treat all” and “treat none” (parallel to the x axis at NB of zero).[10]
Moreover, the favorable strategy is that with the highest clinical NB. Take note of that the unit for NB is the number of true cases discovered per patient and therefore has most extreme esteem at the prevalence π: All cases found, with no false positives.[16]
Code for executing DCA in both R and Stata is accessible from https://www.mskcc.org/departments/epidemiology-biostatistics/health-outcomes/decision-curve-analysis-01.
The R code spares threshold probabilities with the NB for every model; this can then be utilized to diagram the decision curve. The Stata code makes a graph directly, optionally sparing NBs at every threshold as a data set.[17]
In this research, the data were analyzed using SPSS software-version 20, and we have written statistical formula to implement DCA in Microsoft Excel 2013 version.
Results
In this study, the mean age of LBW and NBW infant mothers was approximately 29 years (29.8 ± 4.8 vs. 29.8 ± 5.6) [Table 1]. However, in mothers aged ≤30 years, LBW rate increased 2.17 times which was statistically significant (P < 0.001, odds ratio [OR] = 2.17). With increasing levels of parental education, the LBW percentage increased among mothers so that college-educated mothers (25.3%) or those with college-educated husbands (26.8%) will experience LBW with a higher percentage while it was contrary to uneducated mothers. In addition, 26% of those surveyed were housewives and others were employed women.
Table 1.
Demographic and obstetric characteristics in the two groups
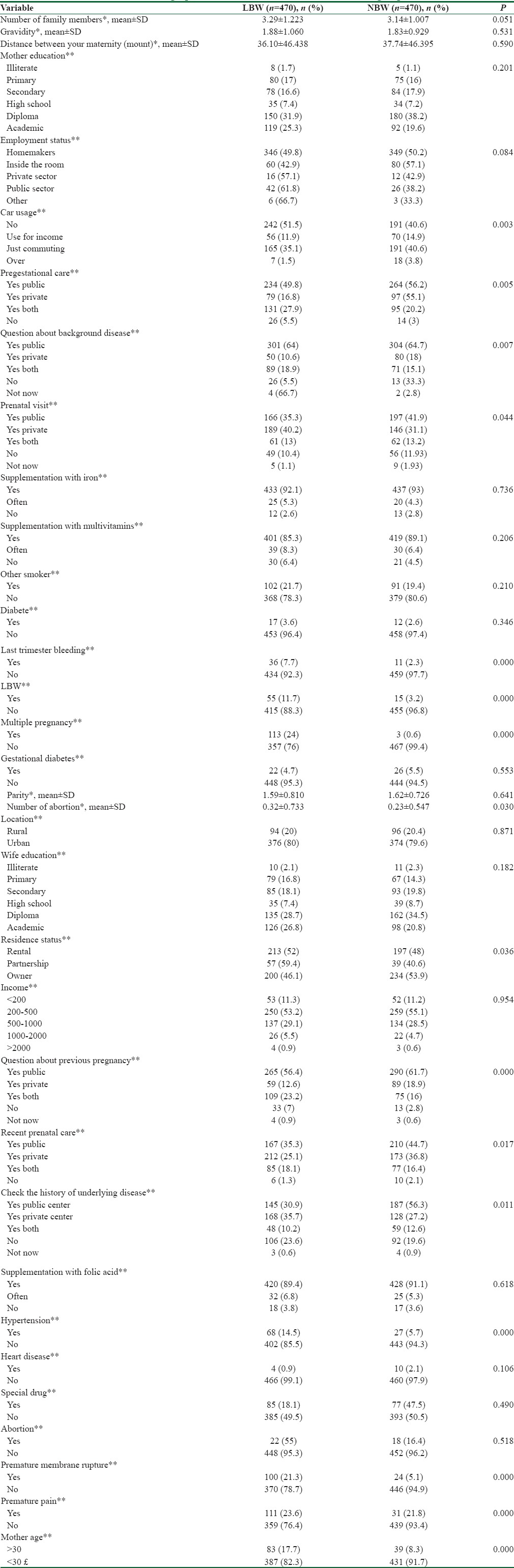
The prevalence of underweight in twin or multiple pregnancy was more than in singleton (24% vs. 0.6%, P < 0.000). LBW infant percentage in the first child (55.1%) was more than other children, and the percentage of LBW decreased with increasing birth rate. The time interval to the recent pregnancy was approximately 36.9 months in both groups of mothers (37.7 vs. 36.1).
Besides, the results of the study showed that most LBW infants were delivered by cesarean (71.9%) and not by normal vaginal delivery (28.1%) which was statistically significant (P < 0.004, OR = 1.5), and some of their mothers had experienced LBW (11.7%) (OR = 2.99, P < 0.002), premature membrane rupture in current pregnancy (21.3%) (OR = 3.18, P < 0.000) and false labor (23.6%) (OR = 2.70, P < 0.000) and last trimester bleeding (5.3%) (OR = 2.58, P < 046).
1.5% of the mothers participating in the study had experienced a history of cardiovascular disease (0.9% vs. 2.1%), 10.1% of them had suffered from hypertension (14.5% vs. 5.7%) in their pregnancy, 20.5% of mothers had experienced passive smoker exposed to tobacco products (21.7% vs. 19.4%), and 5.1% of them had diabetes (4.7% vs. 5.5%) among which the birth of a LBW infant just with hypertension was statistically significant (OR = 2.39, P < 001).
Forty-seven percent of mothers had experienced prenatal cares, counseling and pregnancy history examination and were suffering from underlying diseases, of which 18.7% of these cares had been carried out in the public sector, and the percentage of LBW infants was less among mothers referred to the health centers to receive prenatal cares than those admitted to a private clinics. In addition, 90% of mothers have consumed iron supplements, folic acid, and multivitamins during their pregnancy.
According to the predictor variables, 13 logistic regression models were obtained to identify factors affecting LBW infants’ data. We began from single models (one-variable) and continued to the last model that included 18 variables. Using Hosmer–Lemeshow test statistics (it suggests that the data are well fitted with the model), the significant models (test statistic ≥0.05) were selected, and then, the model with highest Nagelkerke's statistics was selected. This statistic shows the extent the model was able to explain changes of the dependent variable [Tables 2 and 3].
Table 2.
Net benefit obtained from use decision curve analysis on logistic regression prediction models
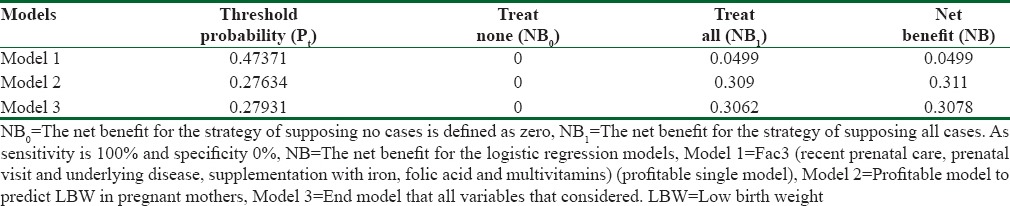
Table 3.
Factors affecting the birth weight as determined by a multiple regression analysis (profitable model)
The threshold probability exists in predictive models, diagnostic tests, and molecular markers and can lead to a variety of decision curves. In the present study, according to the WHO criteria for LBW infant and also considering that the researchers in this study used logistic regression models to identify the most influential variables to predict the LBW infant. The resulting probability for an infant weighing less than 2500 g in this model was used as the threshold probability [Tables 4 and 5].
Table 4.
Relationship between true low birth weight status and result of prediction model with a positivity criterion of 0.2763 predicted probability of low birth weight (profitable model (12) with the highest net benefit)
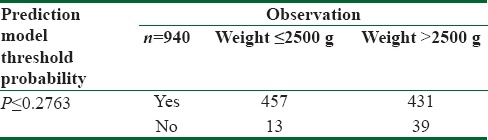
Table 5.
Calculate the net benefit for profitable model and treat all (n=940)

The NB amount in this model was 0.311, which is equivalent to this strategy that can predict 31 LBW infants in 100 cases with no unrealistic reported cases [Figure 1].
Figure 1.
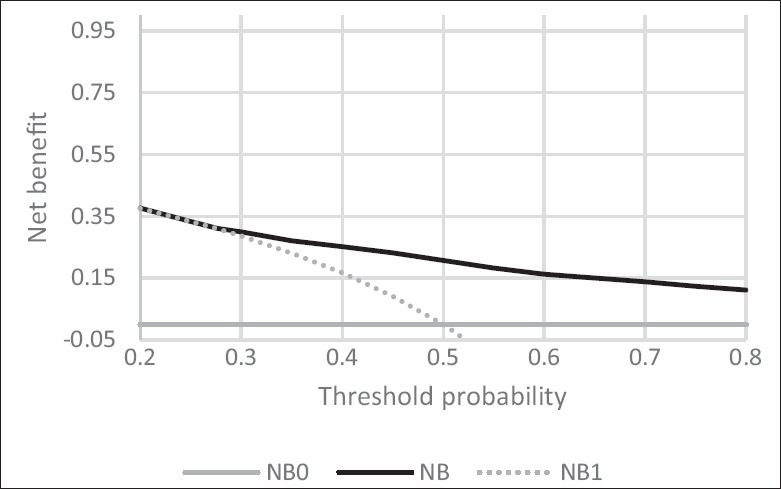
Decision curve analysis for profitable model to predict LBW in pregnant mothers. Solid line: Prediction model. Dotted line: assume all cases be have LBW (all cases are correctly predicted). Thin line: assume no cases be have LBW. The graph gives the expected net benefit per case relative to no LBW in any case (“treat none”). The unit is the benefit associated with report a LBW baby in pregnant mother without any false-positive report. LBW: Low birth weight
Univariate model unlike the best model does not have any desired net profit for our predictor. In addition, since the NB from predictive model (0.049) is equaled with the NB of the treated all model (0.049), an accurate prediction cannot be achieved [Figure 2].
Figure 2.
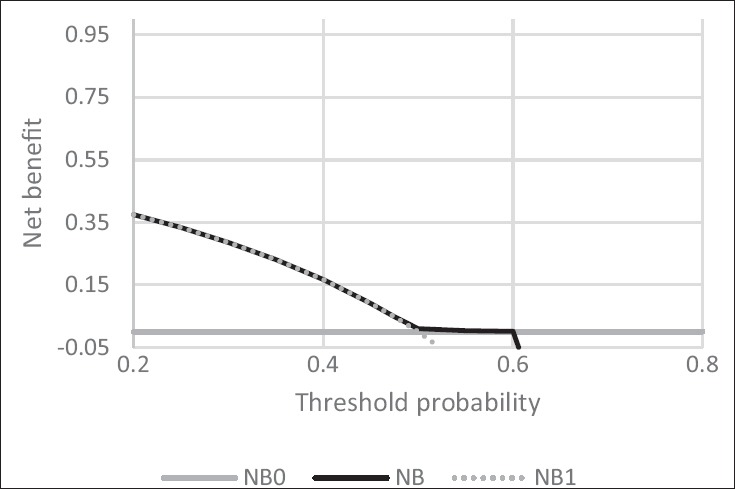
Decision curve analysis for single variable model to predict LBW in pregnant mothers. Solid line: Prediction model. Dotted line: Assume all cases be have LBW. Thin line: Assume no cases be have LBW. LBW: Low birth weight
Discussion
In recent years, with advances in perinatal cares and with setting up Neonatal Intensive Care Unit, survival rate of low-weight and very low-weight premature infants had a significant increase. However, the increased survival of these infants was not concurrent with reducing LBW effects and those babies who survived are more susceptible to problems such as severe disabilities, mental retardation, cerebral palsy, and vision and hearing issues.[18] Social Development Plan (2007–2011) was aimed to reduce the LBW incidence to lower than 7% of all births.[19] The incidence of LBW in Arak Hospital was 9.1% in 2006. It is important to identified risk factors of LBW to protect this problem.[11]
Like different studies from developing countries,[4,12,20,21,22,23,24] the present study has found that danger of having a LBW infant is multifactorial in origin. Numerous maternal, biosocial, medicinal, and obstetric variables contribute to the occurrence of LBW.[25]
Ghavy et al. in a historical cohort study with multiple logistic regression (back Wald and Lemeshow test) found that referred to health centers to receive prenatal cares than those admitted to the public hospital or private clinics/health centers have dependent effect in causing LBW.[12] In this study, the mothers referred to public health centers to receive preconception cares experienced less LBW rate than those referred to private clinics.
Sharma et al. identified that one of the most significant findings to emerge from this study is the maternal age. Analysis by age group revealed a significant positive relationship between age more than 30 years and risk of LBW.[23] In the present study, there was a statistically significant relationship between high maternal age (<30) and an LBW (P < 0.001, OR = 2.17). Besides, this was one of the best predictive logistic regression model variable based on the DCA.
Muchemi et al. in a study entitled, “Factors associated with low birth weight among neonates born at Olkalou District Hospital, Central Region, Kenya” on logistic regression found a significant positive relationship between LBW delivery in a past birth (OR = 4.7, 95% confidence interval [CI] =1.53–14.24), premature membrane rupture (OR = 2.95, 95% CI = 1.14–7.62), premature births (OR = 3.65, 95% CI = 1.31–10.38).[26] In another study by Mirzarahimi et al., they reported that risk of LBW in term newborn children expanded by multiple pregnancy (OR: 3.77, CI: 1.41–10.0), bleeding and spotting (OR: 2.23, CI = 1.22–4.07).[27] The results of this study are consistent with that of the aforementioned studies on the relationship between previous history of LBW (OR = 2.99, P < 0.002), premature membrane rupture (P < 0.000, OR = 3.18), false labor (OR = 2.70, P < 0.000), and last trimester of pregnancy bleeding (P < 0.046, OR = 2.58) and these variables were included as best predictive model variables based on the DCA.
A study from India by Mumbare et al. on logistic regression at revealed that the primary hazard components for SGA recognized were exposure to tobacco, maternal hypertension were associated with delivery of a LBW infants.[22] Another study from India by Jena et al. showed that the maternal factor like passive smoking is significant risk factor for LBW babies.[20] In this study, hypertension during the recent pregnancy and maternal exposure to tobacco products (passive smoker) based on DCA was included as the variables of the Best Predictive Logistic Regression Model.
In a study by Vega et al., multivariate logistic regression analysis demonstrated that some variables: Number of pregnancies, past adverse result, and past LBW were significantly associated with LBW.[24] Moreover, in a same article, Aregay et al. in the first step bivariate analysis were employed to see the association between exposures versus outcome variables. Eventually, factors that are discovered statistically significant under bivariate investigation were entered into multiple logistic regression models to recognize independent predictors of LBW that found gravidity greater than 5 and 1, parity greater than 5 and 1, gestational age <37 weeks, and inter-pregnancy interval (<18 month) were significant maternal risk factors of LBW.[21] In this study, there was not a statistically significant relationship between the LBW infant and the number of pregnancies, the number of previous childbirths, and the time interval with the last delivery or abortion. However, based on DCA, they were included as Predictive Logistic Regression Model variables.
Christian in a double-blind randomized controlled trial that fitted generalized estimating equations binomial regression models found folic acid-iron expanded mean birth weight by 37 g (95% CI: 16–90 g) and lessened the rate of LBW babies (<2500 g) from 43% to 34% (16%; relative risk = 0.84, 0.72–0.99) and multivitamins supplementation expanded birth weight by 64 g (12–115 g) and decreased the rate of low birth weight babies by 14% (0.86, 0.74–0.99).[28] In this study, the use of iron supplements, folic acid, and multivitamins are some of the preventers of LBW infants by a pregnant mother and included as the Best Predictive Model variables with highest NB.
Research to date has been restricted regarding assessment of the capacity of maternal factors to predict the risk of LBW. In spite of huge effect of LBW on neonatal mortality and morbidity, little work has been done to foresee its plausibility only two studies directed in Ohio, USA (proposed a four-figure scale low family working, unpleasant occasions, Quetelet's Index and cigarette smoking) which anticipated LBW that used receiver operating characteristic curve analysis with 65% sensitivity, 84% specificity and 42% PPV[29] and the latter in India (proposed a six–factor scale deficient weight pick up by the mother amid pregnancy [<8.9 kg], insufficient proteins in eating regimen [<47 g/day], past preterm child, past LBW infant, pallid mother and passive smoking) with a sensitivity of 71.58% and specificity of 66.98%.[25]
Utilizing the after effects of record study, the creators used DCA to comparatively assess the maternal parameters concerning their capacity to anticipate LBW delivery and formulated a prediction model or scale to foresee LBW with supplementation with iron, folic acid, and multivitamins during pregnancy, twin or multiple pregnancy, premature membrane rupture, former LBW infants (weighing >2500 g), false labor, hypertension in pregnancy, maternal exposure to tobacco products (passive smoker), last trimester of pregnancy bleeding, a history of cardiovascular disease, mother and spouse's education level, mother age >30, preconception cares, counseling about pregnancy and underlying diseases history in the private sector, history of diabetes, number of pregnancies, and parities that used DCA with 0.3110 NB.
NB has simple clinical interpretation, and this model with NB of 0.3110 at Pt of 0.2763 had profitable among other and utilizing the model is what might as well be called a methodology that distinguished what might as well be called 31.1 LBW per 100 cases with no unnecessary detect.
Other researchers also have used the DCA in their studies. A common point of all these studies is prediction of an outcome using available clinical results. For example, Mr. Vickers and Ms. Elkin used a prostate cancer study data in their research for the introduction of this method. In this study, prostate-specific antigen was used to predict seminal vesicle invasion.[9] Many researches have been carried out on the subject using this model.[13,30,31,32]
Furthermore, Bevevino et al. also used this model in their study entitled “A Model to Predict Limb Salvage in Severe Combat-related Open Calcaneus Fractures” to examine the possibility of amputation of an injured person in the open bone fractures which is a major challenge in making decision for clinician and patient on limb salvage or amputation.[13] Moreover, this method has been widely used for comparing different statistical models in predicting clinical results.[33,34,35,36]
A restriction of this study is that the specimens were taken from hospital center conveyances, and this was a solitary site study. Extra constraints relate to any case–control ponder – i.e., failure to calculate relative risk, unforeseeable risk factor incidence, and memory/recall bias.
Conclusions
It is conceivable to foresee LBW utilizing a prediction model. The biostatistical writing has solely been worried with strategies for assessing the precision of predictive models, diagnostic tests, and molecular markers. While we have to know not just whether a diagnostic test, predictive model or molecular marker is precise, yet whether it is useful clinically. A large portion of the hazard elements for LBW is preventable. This model will do chance stratification of moms and to distinguish those at danger of having a LBW infant. Subsequently, these moms can be alluded amid early pregnancy to a center which is outfitted with facilities for administration of high hazard pregnancy and LBW babies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Hereby, financial support of the Research Department of Isfahan University of Medical Sciences and cooperation of personnel working in health centers and hospitals in the city of Arak and the mothers who helped us in conducting this research are highly appreciated.
References
- 1.Al Habashneh R, Khader YS, Jabali OA, Alchalabi H. Prediction of preterm and low birth weight delivery by maternal periodontal parameters: Receiver operating characteristic (ROC) curve analysis. Matern Child Health J. 2013;17:299–306. doi: 10.1007/s10995-012-0974-2. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF. The State of the World's Children. 2014. Available from: http://www.unicef.org/gambia/SOWC_report_2014.pdf .
- 3.Singh G, Chouhan R, Sidhu K. Maternal factors for low birth weight babies. Med J Armed Forces India. 2009;65:10–2. doi: 10.1016/S0377-1237(09)80045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayode GA, Amoakoh-Coleman M, Agyepong IA, Ansah E, Grobbee DE, Klipstein-Grobusch K. Contextual risk factors for low birth weight: A multilevel analysis. PLoS One. 2014;9:e109333. doi: 10.1371/journal.pone.0109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Heimonen A, Rintamäki H, Furuholm J, Janket SJ, Kaaja R, Meurman JH. Postpartum oral health parameters in women with preterm birth. Acta Odontol Scand. 2008;66:334–41. doi: 10.1080/00016350802307620. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics. Guidelines for Perinatal Care. Washington, DC: American College of Obstetricians and Gynecologists; 1988. [Google Scholar]
- 8.Collins GS, de Groot JA, Dutton S, Omar O, Shanyinde M, Tajar A, et al. External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14:40. doi: 10.1186/1471-2288-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi: 10.1186/1472-6947-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafiei M. Prevalence of low birth weight and obesity and some concomitant factors in live offspring's in 2006 and compare with 2002 result's in Arak Talleghani Hospital. Iran J Pediatr. 2007;17:47–53. [Google Scholar]
- 12.Ghavy A, Soghe KF, Niknamy M, Nejad EK. Maternal factors associated with low birth weight in infants of mothers referred to health centers in Rasht. J Holist Nurs. 2012;15:14–24. [Google Scholar]
- 13.Bevevino AJ, Dickens JF, Potter BK, Dworak T, Gordon W, Forsberg JA. A model to predict limb salvage in severe combat-related open calcaneus fractures. Clin Orthop Relat Res. 2014;472:3002–9. doi: 10.1007/s11999-013-3382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peirce CS. The numerical measure of the success of predictions. Science. 1884;4:453–4. doi: 10.1126/science.ns-4.93.453-a. [DOI] [PubMed] [Google Scholar]
- 15.Rousson V, Zumbrunn T. Decision curve analysis revisited: Overall net benefit, relationships to ROC curve analysis, and application to case-control studies. BMC Med Inform Decis Mak. 2011;11:45. doi: 10.1186/1472-6947-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers AJ. Decision analysis for the evaluation of diagnostic tests, prediction models and molecular markers. Am Stat. 2008;62:314–20. doi: 10.1198/000313008X370302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Vickers AJ. Decision curve analysis: A discussion. Med Decis Making. 2008;28:146–9. doi: 10.1177/0272989X07312725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soleimani F, Zaheri F, Abdi F. Developmental outcome of low birth-weight and preterm newborns: A re-view of current evidence. Tehran Univ Med J. 2013;71:551–61. [Google Scholar]
- 19.Kanjanasingh T. The association between antenatal care and low birth weight newborn at. R Thai Airforce Med Gaz. 2014;59:9–13. [Google Scholar]
- 20.Jena SK, Mishra S, Swamy G. A study of risk factors associated with low birth weight babies born to mothers attending a tertiary hospital of Andhra Pradesh. Indian J Public Health Res Dev. 2015;6:277–81. [Google Scholar]
- 21.Aregay A, Wendafrash M, Berhe S. Maternal risk factors associated with low birth weight in Tigray Region, Northern Ethiopia, 2010. Int J Nurs Didactics. 2015;5:11–20. [Google Scholar]
- 22.Mumbare SS, Maindarkar G, Darade R, Yenge S, Tolani MK, Patole K. Maternal risk factors associated with term low birth weight neonates: A matched-pair case control study. Indian Pediatr. 2012;49:25–8. doi: 10.1007/s13312-012-0010-z. [DOI] [PubMed] [Google Scholar]
- 23.Sharma MK, Kumar D, Huria A, Gupta P. Maternal risk factors of low birth weight in Chandigarh India. [Last accessed on 2010 Dec 10];Internet J Health. 2009 9 Available from http://www.ispub.com/journal/the_internet_journal_of_health/volume_9_number_1_12/article/maternal-risk-factors-of-low-birthweight-in-chandigarh-india.html . [Google Scholar]
- 24.Vega J, Sáez G, Smith M, Agurto M, Morris NM. Risk factors for low birth weight and intrauterine growth retardation in Santiago, Chile. Rev Med Chil. 1993;121:1210–9. [PubMed] [Google Scholar]
- 25.Singh A, Arya S, Chellani H, Aggarwal KC, Pandey RM. Prediction model for low birth weight and its validation. Indian J Pediatr. 2014;81:24–8. doi: 10.1007/s12098-013-1161-1. [DOI] [PubMed] [Google Scholar]
- 26.Muchemi OM, Echoka E, Makokha A. Factors associated with low birth weight among neonates born at Olkalou District Hospital, Central Region, Kenya. Pan Afr Med J. 2015;20:31. doi: 10.11604/pamj.2015.20.108.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirzarahimi M, Hazrati S, Ahmadi P, Alijahan R. Prevalence and risk factors for low birth weight in Ardabil, Iran. Iran J Neonatol. 2013;4:18–23. [Google Scholar]
- 28.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: Double blind randomised community trial. BMJ. 2003;326:571. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeb KG, Graham AV, Zyzanski SJ, Kitson GC. Predicting low birthweight and complicated labor in urban black women: A biopsychosocial perspective. Soc Sci Med. 1987;25:1321–7. doi: 10.1016/0277-9536(87)90130-4. [DOI] [PubMed] [Google Scholar]
- 30.Lundon DJ, Kelly BD, Foley R, Loeb S, Fitzpatrick JM, Watson RW, et al. Prostate cancer risk assessment tools in an unscreened population. World J Urol. 2015;33:827–32. doi: 10.1007/s00345-014-1365-7. [DOI] [PubMed] [Google Scholar]
- 31.Hansen J, Auprich M, Ahyai SA, de la Taille A, van Poppel H, Marberger M, et al. Initial prostate biopsy: Development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. 2013;63:201–9. doi: 10.1016/j.eururo.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Guazzoni G, Lazzeri M, Nava L, Lughezzani G, Larcher A, Scattoni V, et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer. Eur Urol. 2012;61:455–66. doi: 10.1016/j.eururo.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Forsberg JA, Sjoberg D, Chen QR, Vickers A, Healey JH. Treating metastatic disease: Which survival model is best suited for the clinic? Clin Orthop Relat Res. 2013;471:843–50. doi: 10.1007/s11999-012-2577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyce S, Fan Y, Watson RW, Murphy TB. Evaluation of prediction models for the staging of prostate cancer. BMC Med Inform Decis Mak. 2013;13:126. doi: 10.1186/1472-6947-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lughezzani G, Zorn KC, Budäus L, Sun M, Lee DI, Shalhav AL, et al. Comparison of three different tools for prediction of seminal vesicle invasion at radical prostatectomy. Eur Urol. 2012;62:590–6. doi: 10.1016/j.eururo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cammann H, Jung K, Meyer HA, Stephan C. Avoiding pitfalls in applying prediction models, as illustrated by the example of prostate cancer diagnosis. Clin Chem. 2011;57:1490–8. doi: 10.1373/clinchem.2011.166959. [DOI] [PubMed] [Google Scholar]


