Abstract
Background:
The purpose of this study was to evaluate the microbial reduction in deciduous molars using Morinda citrifolia juice (MCJ) as irrigating solution.
Materials and Methods:
This was a randomized comparative study including 60 deciduous molars chosen among the patients belonging to the age group of 6–9 years based on the inclusion or exclusion criteria. The selected teeth were divided randomly into two groups based on irrigation solution used, that was, Group I (1% NaOCl) and Group II (MCJ). The microbial samples were collected both pre- and post-irrigation and were transferred for microbial assay. Paired t-test was used for intragroup analysis of pre- and post-operative mean reduction of bacterial colony forming unit (CFU)/ml, whereas Independent t-test was used to assess the intergroup, pre- and post-operative mean reduction of bacterial CFU/ml.
Results:
In the intragroup comparison, both of the groups showed statistically significant (P < 0.001) reduction in the mean CFU/ml; however, it did not show statistically significant reduction when intergroup comparison was carried out between the two groups. Both the study materials had clinically revealed decrease in the microbial count postirrigation.
Conclusion:
Both the irrigants, 1% NaOCl and MCJ, were significantly effective in the reduction of mean CFUs/ml postoperatively. The results of this study have confirmed the antibacterial effectiveness of MCJ in the root canals of deciduous teeth. Considering the low toxicity and antibacterial effectiveness of MCJ, it can be advocated as a root canal irrigant in endodontic treatment of primary teeth.
Key Words: Deciduous, molar, plant extracts, sodium hypochlorite
INTRODUCTION
Maintaining the integrity of the primary dentition until the eruption of permanent teeth is of the utmost importance for proper growth and development of the facial skeleton complex. However, deep carious lesions create conditions mandating endodontic treatment. It is indeed tedious to perform endodontic treatment in deciduous teeth due to complex root canal morphology, proximity of the developing successors superadded to difficulties related to behavior management in children.[1,2,3]
The successful outcome of endodontic treatment depends on through disinfection. Based on this concept, the ultimate goal of endodontic therapy is to eliminate or remarkably minimize the microbes and their toxic products from the root canal before obturation.[4] However the complex morphology of root canal system, invasion of microbes into the dentinal tubules, and formation of smear layer during instrumentation are the major obstacles in achieving complete cleaning and shaping of the root canals.[5]
The role of mechanical instrumentation alone in removing microbes from the root canals has been investigated in many studies, and majority of them have found a significant reduction in the microbial count with an increasing preparation size and irrigation[6] However, Peter and Wesselink[7] found that more than 35% of root canal walls remained untouched even by modern rotary Ni-Ti instrumentation techniques.
Adequate cleaning of the root canal system in deciduous teeth involves both the chemical and mechanical methods to avoid the overzealous and undesired removal of dentin. Mechanical instrumentation establishes a specific cavity form, creating easy access for the instruments and irrigating solutions into the root canal space. Irrigating solutions are mandated to eradicate the microorganisms, and a variety of chemicals have been used for this purpose over a period of time. The ideal irrigant kills the bacteria, dissolves necrotic tissue, lubricates the canal, removes the smear layer, and does not irritate healthy tissues.[8]
Irrigants currently being advocated during cleaning and shaping in day-to-day clinical practice can be divided as antibacterial and decalcifying agents or their combinations. They include sodium hypochlorite; chlorhexidine; ethylenediamine tetra-acetic acid (EDTA); and a mixture of tetracycline, an acid, and a detergent. However, there is no unique irrigant that fulfills the properties of an ideal irrigant for deciduous or permanent dentition. Sodium hypochlorite (NaOCl) is the most commonly used endodontic irrigating solution in dilutions ranging from 0.5% to 5.25%. Advantages of NaOCl include its ability to dissolve organic substances in the root canal system and its affordability. However, its major disadvantages are cytotoxicity when accidently injected into periradicular tissues, foul smell and taste, ability to bleach clothes, and ability to corrode metal object.[9]
Literature has documented various plant extracts having antimicrobial and therapeutic properties which can serve the purpose of endodontic irrigation. Morinda Citrifolia, commonly known as noni which is a tree in the coffee family, Rubiaceae. M. Citrifolia Juice (MCJ) has a broad range of therapeutic effects including antibacterial, antiviral, antifungal, antitumor, anthelmintic, analgesic, hypotensive, anti-inflammatory, and immune enhancing effects.[10] Therefore, the aim of this study was to evaluate the microbial reduction in deciduous molars using MCJ as irrigating solution.
MATERIALS AND METHODS
This study was a randomized comparative study conducted in patients who presented for routine endodontic therapy in the Department of Pedodontics and Preventive Dentistry. Ethical clearance for the same was obtained from the institutional ethical committee. A total of 60 cases of deciduous molars of 6–9-year-old children with no systemic and medical conditions and who were not on any antibiotics/anti-inflammatory drugs for at least 3 months before were included based on following findings.
-
Clinical finding
- Adequate coronal structure to support rubber dam
- Multi-rooted tooth with necrotic pulp, abscess, or sinus tract
-
Radiographic findings
- Radiolucency involving enamel, dentin, and pulp in multi-rooted teeth
- Teeth having at least 2/3 of root length
- Furcation involvement in multi-rooted tooth
- Multi-rooted teeth with periapical rarefaction.
The selected tooth samples were randomly divided into two groups of 30 for irrigation. Group I-1% NaOCl and Group II-MCJ. The desired concentration of irrigants was achieved by diluting them with distilled water.
Before starting the study, minimum inhibitory concentration of MCJ was performed using the broth dilution method.
After obtaining informed consent from legal guardian, local anesthesia was administered and the offending tooth was isolated using rubber dam. The endodontic field was swabbed using povidone-iodine for 3 min to eliminate surface contaminants and then washed with sterile saline solution, following which 5% sodium thiosulfate solution was used to inactivate the iodine tincture so that its remnants would not affect the process of microbial sampling.[11]
Access opening was made using a round bur in high speed, de-roofing was done and coronal pulp removed. The canals were located and pulp tissue was extirpated from root canals using Barbed Broach following which the working length was established by the Ingle's radiographic method. Samples were collected from the palatal canal in the maxillary molars and distal canal in mandibular molars. The contents of the canal were absorbed into sterile paper points at the most apical extent of the canal for 60 s; the saturated paper points were deposited into 10 ml of thioglycollate broth for anaerobic culture.[12] This preoperative sample (S1) was taken to confirm the presence of bacteria in the root canal system. The samples were labeled and transported to the microbiology laboratory within 15 min.
Chemico-mechanical preparation was done using stainless steel H– files till size 30/35 followed by irrigation with 3 ml of one of the irrigants. Following cleaning, shaping, and irrigation, postoperative samples (S2) were collected following the same protocol as that for preoperative sample and transported to microbiology laboratory within 15 min.
For both the groups after final irrigation with normal saline, the canals were dried with absorbent paper points and obturated with zinc oxide eugenol by means of hand pluggers to push the paste just short of the apex. The coronal space was filled with Type II GIC cement.
Microbiological processing
The pre- and post-operative samples (paper points) placed in 10 ml of thioglycollate broth was vortexed in a vortex mixer (Eltek VM 301) for 1 min. Dilutions of 10, 100, 1000, 10,000, 100,000-fold were made. Serial dilution was carried out as follows.
A volume of 1 ml of transport medium containing sample was transferred to the vial containing 9 ml of sterile peptone water bringing in 10−'2 dilution. Then, 1 ml from this vial was transferred to the next vial containing 9 ml of sterile peptone water to make it a 10−'3 dilution. This procedure was continued in similar way up to 10−'5 dilution.
The blood agar plates were inoculated with 0.1 ml of undiluted sample (10 dilution) as well as each of the four dilutions with the help of sterile spreaders. For anaerobic samples, nutrient agar plates were kept in GasPak jar and incubated for up to 48 h. The growth was observed in each medium after respective incubation. The colonies were counted next day using microbial colony counter (HiMedia).
Statistical analysis
All the collected group-wise data were entered and computed by statistical software SPSS version 16. A log10 transformation of each colony forming unit (CFU) count was done to normalize the data before the statistical evaluation due to high variance of bacterial numbers. Paired t-test was used to detect the intragroup significance in the reduction of bacteria from preoperative sample to post-operative sample and for intergroup comparison; Independent sample t-test was used. The level of significance was same at 0.05 for all analysis.
RESULTS
Table 1 illustrates the pre- and post-operative bacterial CFU of Group I and II, respectively. Table 2 shows that mean CFU/ml preoperative was 4.77 in 1% NaOCl group. There was a significant reduction in colony counts to 2.34 postoperative (P < 0.001). Furthermore, the mean CFU per ml preoperative was 4.78 in MCJ group [Table 2]. Postoperatively, there was a significant reduction in colony counts to 2.39 (P < 0.001). Table 3 shows that mean overall CFU per ml preoperative in 1% NaOCl group and MCJ group was 4.754 and 4.758, respectively. Postoperatively, in 1% NaOCl group, mean CFU per ml decreased to 2.34, whereas in MCJgroup it reduced to 2.39. However, there was no significant difference in mean CFU per ml pre- and post-operative between 1% NaOCl group and MCJ group (P > 0.05).
Table 1.
Pre- and post-operative bacterial count CFUs in Group 1 (1% sodium hypochlorite) and Group II (Morinda citrifolia juice)
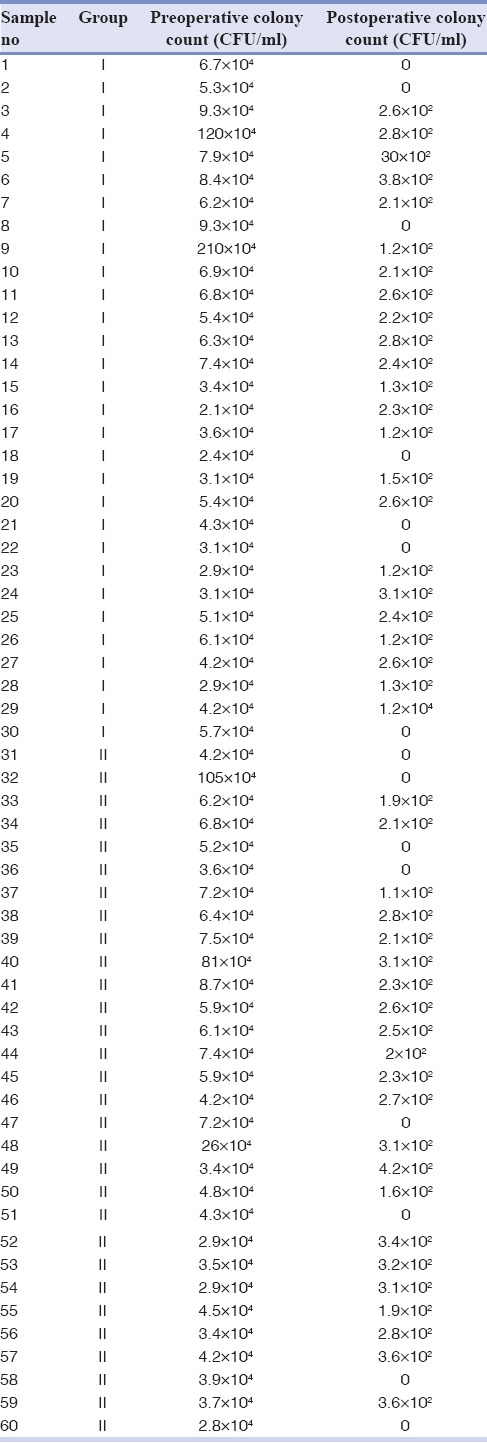
Table 2.
Intragroup comparison of overall mean CFU/ml pre- and post-operative in 1% sodium hypochlorite group and Morinda citrifolia group
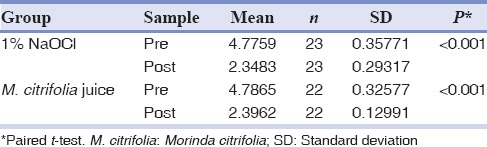
Table 3.
Intergroup comparison of overall mean CFU/ml of between 1% sodium hypochlorite group and Morinda citrifolia juice group
DISCUSSION
The most crucial aspect of the endodontic treatment is the complete eradication of bacteria from the root canal system, and in deciduous teeth, it is more tedious due to ribbon-shaped and tortuous root canal proximity to the developing successor along with difficulty in behavior management in children.[1,2,3]
To remove the potentially infected pulp tissue and dentinal debris from the root canal system, it is necessary to use copious irrigation solution simultaneously with instrumentation during canal preparation, as it has been reported that irrespective of the file system used, instrumentation alone leaves more than 35% of root canal walls untouched.[7]
Many irrigating solutions have been studied extensively to determine the one which best exhibit the ideal properties, but the ideal irrigant has not yet been realized.
Sodium hypochlorite commonly termed as gold standard irrigant, can meet many of the criteria of an ideal irrigant. It has a broad antibacterial spectrum possessing even the ability to inactivate endotoxin and unlike other irrigants, it is effective at dissolving tissue and removing the organic component of the smear layer.[9] In day-to-day dental practice, it is used in dilutions ranging from 0.5% to 5.25%. Sirtes et al.[13] found the capacity of a1% NaOCl at 45°C to dissolve human dental pulps equal to that of a 5.25% solution at 20°C. Whereas, Zou et al.[14] found that temperature, time and concentration all contribute to the penetration of sodium hypochlorite into dentinal tubules. It is evident from the recent available facts that there is no justification for using NaOCl at concentrations higher than 1% as this achieves the desirable clinical effects.[15] Taking all these proved properties into consideration it was decided to use 1% NaOCl as a standard control group.
However, everything has its advantages and disadvantages. Although NaOCl has a wide spectrum of useful properties it has disadvantages too, including its caustic nature, tissue damage risks if NaOCl is expressed under pressure into the periodontal ligament space and also reduction in flexural strength of dentin.[9] Hence, there is always a quest for an alternative effective and easily available irrigant that fulfills all the criteria with minimal possible side effects.
Literature has shown that certain natural plant extracts have antimicrobial and therapeutic effects suggesting its possibility be used as an endodontic irrigant.
M. Citrifolia, commonly known as Noni is a member of the coffee family, Rubiaceae having a broad range of therapeutic effects, including analgesic, hypotensive, antibacterial, antiviral, antifungal, antitumor, antihelmintic, anti-inflammatory, and immune-enhancing effects. Antibacterial effect of MCJ is mainly attributed to the compounds L-asperuloside and alizarin.[16] Using MCJ as an endodontic irrigant might be advantageous because of its biocompatible, antioxidant, and nontoxic nature.[17] Considering the above-mentioned advantages, it was decided to use MCJ as the test group.
In this study, the minimum inhibitory concentration of MCJ on Enterococcus faecalis growth in vitro was established to be a 7% solution. Thereafter, a 7% solution of MCJ diluted in distilled water was used in comparison with 1%NaOCl.
The tooth surface was disinfected using 5% povidone-iodine – a simple effective method proven to be efficacious and widely used.[11]
Grossman et al.[12] advocated the use of sterile paper points for obtaining the root canal samples and advocated that 1–10 microorganisms are optimum for obtaining growth through culture procedures. Whereas, Molander et al.[18] pointed out the propagation of microorganisms throughout the entire root canals of deciduous teeth, including the lumen, accessory canals, and dentinal tubules; apical delta, apical foramen and periapical biofilm. Considering both these aspects, microbial samples were collected from the most difficult area to be cleaned, that is, the apical third of the canal absorbent paper points were used for collection of microbiological samples as it collects bacteria present only inside the canal and not from the dentinal tubules or deeper areas, thus reducing the chances of variability in quantity and type of microbial isolation from the root canal system.
In the current study, both the groups showed statistically significant reduction in the bacterial population on intragroup comparison, that is, preoperative mean CFU/ml counts Vs postoperative mean CFU/ml counts in each group using paired sample t-test [Table 2]. Thus, it can be stated that a significant lower mean CFU counts at postoperative phase implies that both the study materials were potent in bacterial reduction. However, when overall mean cfu/ml between Group I and II was calculated using independent sample t-test [Table 3] no significant difference in mean CFU per ml pre- and post-operative was obtained (P > 0.05). The results are in accordance with Murray et al.[17] who demonstrated that efficacy of MCJ was similar to NaOCl in conjunction with EDTA Pandranki et al.[19] who assessed antimicrobial efficacy of the two Morinda species at different concentration against endodontic microorganisms following agar well-diffusion assay founded a statistically significant difference in the antimicrobial efficacy of M. citrifolia and Morinda tinctoria at different concentrations as compared to 3% NaOCl.
None of the protocols tested in this study showed 100% reduction of the microbes from root canal system. In the intragroup comparison, both the groups showed statistical significant (P < 0.001) reduction in the mean CFU/ml; however, it did not reach statistically significant values when intergroup comparison was carried out between the two groups. Both the study materials had clinically demonstrated decrease in the organisms status postirrigation.
Literature regarding the use of irrigants in deciduous teeth is sparse and still blooming particularly in the case of herbal products as endodontic irrigants.[20,21] In the present randomized comparative study, evaluating the efficacy of a herbal product, that is, MCJ in comparison to 1% NaOCl, both the irrigants had demonstrated substantial reduction in the microbial count postirrigation which can be further augmented by increasing the quantity of irrigant and adhering to multi-visit protocol for pulpectomy.
There are limitations to this study. Further studies with a larger sample size for each treatment group could better confirm the findings of this study and which combines PCR as well as bacterial CFU colony counts could possibly better confirm true bacterial populations.
CONCLUSION
To conclude, both the irrigants, 1% NaOCl and MCJ, were significantly effective in the reduction of mean CFUs/ml postoperatively. However, on the intergroup comparison, there was no statistically significant difference observed in the antimicrobial efficacy between 1% NaOCl and MCJ.
To the best of our knowledge, this is the first in vivo comparison between the 1% NaOCl and MCJ. Despite the lack of significant differences, this study provides valuable preliminary data and a framework for future clinical studies. Within the limits of the present study, it can be stated that MCJcan serve as alternative available natural extract for irrigation in day-to-day routine dental clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Chawla HS, Mani SA, Tewari A, Goyal A. Calcium hydroxide as a root canal filling material in primary teeth – A pilot study. J Indian Soc Pedod Prev Dent. 1998;16:90–2. [PubMed] [Google Scholar]
- 2.Paras LG, Rapp R, Piesco NP, Zeichner SJ, Zullo TG. An investigation of accessory foramina in furcation areas of human primary molars: Part 1.SEM observations of frequency, size and location of accessory foramina in the internal and external furcation areas. J Clin Pediatr Dent. 1993;17:65–9. [PubMed] [Google Scholar]
- 3.Seow WK. The effects of dyadic combinations of endodontic medicaments on microbial growth inhibition. Pediatr Dent. 1990;12:292–7. [PubMed] [Google Scholar]
- 4.Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20:276–8. doi: 10.1016/s0099-2399(06)80815-0. [DOI] [PubMed] [Google Scholar]
- 5.Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:658–66. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 6.Hülsmann M, Hahn W. Complications during root canal irrigation – Literature review and case reports. Int Endod J. 2000;33:186–93. doi: 10.1046/j.1365-2591.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660–7. doi: 10.1046/j.1365-2591.2002.00541.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters OA, Peters CI. Cleaning and shaping of the root canal system. In: Hargreaves K, Cohen S, editors. Cohen's Pathways of Pulp. 10th ed. St. Louis.: Elsevier Mosby Publication; 2011. pp. 283–341. [Google Scholar]
- 9.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 10.Penumudi SM, Mandava RB, Saraswathi DD, Santhi V, Bollineni S, Gandhi B. Antimicrobial efficacy of herbs in endodontics. J Adv Oral Res. 2015;6:44–7. [Google Scholar]
- 11.Onçag O, Hosgör M, Hilmioglu S, Zekioglu O, Eronat C, Burhanoglu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 12.Grossman LI, Oliet S, Del Rio CE, editors. Endodontic Practice. 11th ed. Philadelphia: Lea & Febiger; 1988. Microbiology; pp. 234–41. [Google Scholar]
- 13.Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005;31:669–71. doi: 10.1097/01.don.0000153846.62144.d2. [DOI] [PubMed] [Google Scholar]
- 14.Zou L, Shen Y, Li W, Haapasalo M. Penetration of sodium hypochlorite into dentin. J Endod. 2010;36:793–6. doi: 10.1016/j.joen.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Good M, El KI, Hussey DL. Endodontic 'solutions' part 1: A literature review on the use of endodontic lubricants, irrigants and medicaments. Dent Update. 2012;39:239. doi: 10.12968/denu.2012.39.4.239. [DOI] [PubMed] [Google Scholar]
- 16.Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, Palu AK, et al. Morinda citrifolia (Noni): A literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002;23:1127–41. [PubMed] [Google Scholar]
- 17.Murray PE, Farber RM, Namerow KN, Kuttler S, Garcia-Godoy F. Evaluation of Morinda citrifolia as an endodontic irrigant. J Endod. 2008;34:66–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 19.Pandranki J, Narasimha Rao VV, Vijayalakshmi G, Dorothy KP. Ethnobotanical approach against resistant endodontic pathogens using Morinda species – An antimicrobial study. Int J Biol Med Res. 2013;4:3661–6. [Google Scholar]
- 20.Verma MK, Pandey RK, Khanna R, Agarwal J. The antimicrobial effectiveness of 25% propolis extract in root canal irrigation of primary teeth. J Indian Soc Pedod Prev Dent. 2014;32:120–4. doi: 10.4103/0970-4388.130786. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Kenchappa M, Gupta P, Chaurasiya S, Sharma P, Satyarth S. Smear layer removal in primary teeth using a novel irrigant, QMix: An in vitro study. J Cranio Maxillary Dis. 2015;4:137–43. [Google Scholar]


