Abstract
Background:
Oral cancer has one of the highest mortality rate among other malignancies. An attempt has been made to assess the genetic expression of a cell surface glycoprotein component - sialic acid released by the malignant cells which will reflect on the oral squamous cell carcinoma (OSCC). The aim of this study was to estimate and correlate the salivary and serum sialic acid levels in OSCC.
Materials and Methods:
In our case–control study, saliva and blood samples were obtained from Group 1 - 10 healthy controls, Group 2 - 12 well-differentiated OSCC, Group 3 - 7 moderately differentiated and 2 poorly differentiated OSCC. Serum and salivary total sialic acid levels were analyzed by Warren's thiobarbituric acid method and acidic ninhydrin method, respectively. The results were analyzed statistically by Student's t-test and Pearson's correlation coefficient (P ≤ 0.05).
Results:
A significant difference in the serum and salivary sialic acid levels was observed between Group 1 and Group 3 (P = 0.01 and < 0.0001) and in salivary sialic acid between Group 2 and Group 3 (P = 0.02). A significant positive correlation was observed between salivary and serum sialic acid in Groups 2 and 3 together (P = 0.015).
Conclusion:
As the histopathological grade progresses, there is a marked increase in level of sialic acid. There is a significant positive correlation between serum and salivary sialic acid levels in OSCC. Further research with larger sample size along with grading and staging system may highlight its significance in OSCC.
Key Words: Biomarkers, carcinoma, N-acetylneuraminic acid, saliva, serum
INTRODUCTION
Head and neck cancer accounts for 30%–40% of all malignant tumors in India,[1] and the most common malignant neoplasm is oral squamous cell carcinoma (OSCC).[2] The World Health Organization has ranked oral cancer as one of the highest mortality rates among the other malignancies in spite of its recent advances in treatment. In 45% of cases, death occurs within 5 years from diagnosis. This can be prevented with early diagnosis and treatment.[3]
Oral cancer results from the accumulation of multiple genetic changes in the cells due to sustained exposure to carcinogens such as tobacco and ethanol. Hereditary factors and viral infections, lifestyle, and dietary factors also contribute to the risk factors of oral cancer.[4]
The plasma membrane is one of the sites where genetic changes are expressed and is involved in the control of normal cell behavior and proliferation. It is composed of phospholipids, glycoproteins, and glycolipids. The nonreducing end of the carbohydrate chains of these glycoproteins and glycolipids is predominantly of sialic acid. Sialic acid belongs to a family of acylated derivatives of neuraminic acid.[5,6] It plays an important role in cell–cell recognition, invasiveness, adhesiveness, and immunogenicity.[6,7,8,9]
Transformed cells often show altered content of sialic acid on their surface glycoconjugates and this alteration seems to be an early event in the tumorigenesis.[7] Furthermore, there is an elevation in the level of sialic acid on the cell surface. These glycoconjugates are released into the circulation through increased turnover, secretion, and/or shedding from malignant cells, leading to increase in the sialic acid levels in blood and other body fluids.[6]
Numerous biochemical markers such as p53, antibody to p53, and salivary defensin have been studied for determining early diagnosis and prognosis of OSCC. At present, no single tumor marker can validate the presence or prognosis of the disease.[10] Sialic acid (N-acetylneuraminic acid) is one such biochemical marker which has been reported to be elevated in sera of patients with a range of malignancies. This study is undertaken to estimate and correlate the salivary and serum sialic acid levels in OSCC.
MATERIALS AND METHODS
The study subjects for our case–control study were procured from Mahatma Gandhi Postgraduate Institute of Dental Sciences, Pondicherry. All the participants were informed about the procedure and written consent was obtained. Ten healthy controls (Group 1) who underwent routine blood investigation for other dental treatments with no systemic illness or recent local illness and without any adverse habits were included in the study. Twenty-one patients with OSCC who were histopathologically graded (Broder's grading[11] system) as 12 well-differentiated (Group 2), seven moderately differentiated, and two poorly differentiated (Group 3) were included in the study. Patients who have undergone treatment for OSCC or who have potentially malignant disorders, cardiovascular diseases, or any other conditions which may change the sialic acid levels were excluded from the study group. The study was approved by the Institutional ethical committee (I-198/MGPGI/Aca/A5/2010-11/1947).
Samples were collected between 9 a.m. and 11 a.m. Two milliliters of blood sample was collected by venous arm puncture, and the serum was obtained through centrifugation. Sialic acid was estimated by Warren's thiobarbituric acid method,[12] and were read in a colorimeter at 540 nm.
Two milliliters of whole unstimulated salivary samples were collected by spitting the pooled saliva into a sterile container (by Navazesh method[13]) between 9 a.m. and 11 a.m. Salivary samples were centrifuged at 3000 rpm for 15 min, and the obtained supernatant was used for the estimation of salivary total sialic acid using acidic ninhydrin method.[14] Optical density (OD) was measured at 480 nm using the colorimeter.
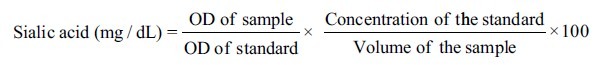
Where, OD is the optical density.
Statistical analysis
The data were analyzed and the values of biochemical parameters were expressed as mean ± standard deviation (SD); the levels of significance were determined by Student's t-test. Pearson's correlation coefficient was performed to correlate serum and salivary total sialic acid levels.
RESULTS
Table 1 shows the mean and SD of salivary and serum sialic acid levels. Table 2 compares the salivary and serum sialic acid levels of Group 1 with Group 2 and Group 3. Table 3 compares the salivary and serum sialic acid levels among Group 1, Group 2, and Group 3. Statistically, no significance was found between Group 1 with Group 2 and Group 3. A significant difference was recorded in salivary and serum sialic acid level between Group 1 and Group 3. Furthermore, a significant difference was found in salivary sialic levels between Group 2 and Group 3. We found a significant positive correlation between salivary and serum sialic acid levels in Groups 2 and 3 together. There is an observable correlation between serum and salivary sialic acid levels in Group 3. However, there is no statistically significant correlation between serum and salivary sialic acid levels in Group 1 and Group 2 [Table 4].
Table 1.
Salivary and serum sialic acid level in all the groups
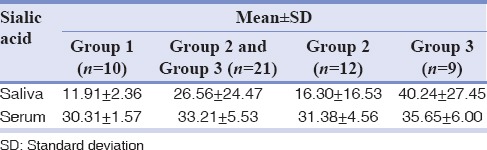
Table 2.
Comparison of salivary and serum sialic acid level of Group 1 with Group 2 and Group 3

Table 3.
Comparison of salivary and serum sialic acid level between Groups 1, 2, 3
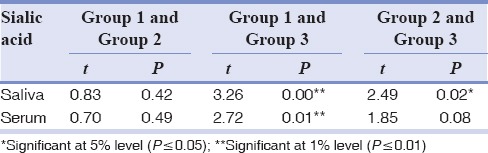
Table 4.
Pearson's correlation coefficient between serum and salivary sialic acid levels

DISCUSSION
Cancer is the second leading cause of death worldwide. India has the highest incidence of OSCC.[4] Cancer, when diagnosed early, has a survival rate of 80%–90%.[3] The plasma membrane consists of oligosaccharide chains of glycoproteins, glycolipids, and phospholipids. The terminal ends of these oligosaccharide chains contain sialic acid along with other modified monosaccharides.[15]
Cell activation, transformation, and malignant growth increase the spontaneous shedding of cell surface components. In growing cells, the rate of carbohydrate synthesis is significantly higher compared with nongrowing cells. Consequently, this shedding process is a normal event linked to the activation and growth periods of the normal cell; however, in cancer cells, shedding is a continuous and rapidly on-going phenomenon. Increased sialylation helps malignant cells to disguise their immunogenic sites, to increase the negative charge of the outer cell membrane so that the binding and killing by lymphocytes and macrophages can be impaired, and to hide the receptor sites for IgM antibodies, which kill cells by a complement-mediated reaction.[8]
It is elevated in sera of patients with a range of malignancies such as cancers of oral cavity, ovary, endometrium, stomach, breast, colorectum, gall bladder, and thyroid.[16]
Saliva is a unique fluid which can be used as a diagnostic tool. The anatomical proximity of saliva to malignant oral neoplasm makes it ideal for screening.[17] Sialic acid is also reported in saliva, urine, meconium, brain, milk, colostrums, mammary glands, etc.[18]
In the present study, grouping of OSCC was done based on Broder's histopathological grading system[11] since it represents the individual cell's maturity level which may correlate with the cell's aggressiveness. Due to inadequacy in availability of cases, moderately and poorly differentiated squamous cell carcinoma cases being at higher level in Broder's grading system were grouped together as single group to obtain appropriate sample size. The mean total salivary sialic acid level in controls was found to be 11.91 mg/dl and in well-differentiated and moderately/poorly differentiated groups were found to be 16.30 mg/dl and 40.24 mg/dl, respectively. The difference in the level of total sialic acid in saliva of OSCC patients was found to be statistically insignificant compared with the control group. This is in contrast with the studies of Shivashankara and Prabhu[4] and Sanjay et al.,[10] wherein elevated salivary sialic acid levels were found in OSCC compared with the healthy individuals. This may be due to the difference in sampling, i.e., number of patients with different histopathological grades of OSCC. The mean serum sialic acid level in controls was found to be 30.31 mg/dl, which correlates with that of Yadav et al.[19]
The mean serum sialic acid level in well-differentiated and moderately/poorly differentiated group was found to be 31.38 mg/dl and 35.65 mg/dl, respectively. In a previous study by Joshi and Patil,[1] the mean serum sialic acid level in well-differentiated, moderately differentiated, and poorly differentiated groups was found to be 83.84 mg/dl, 82.73 mg/dl, and 95.02 mg/dl, respectively. This variation might be due to the difference in the method applied in the estimation of sialic acid.
Statistically, significant difference was not observed in the level of total sialic acid in serum between control and OSCC group which is in accordance with the studies by Shantaram et al.[9] This is in contrast to the studies of Joshi and Patil,[1] Raval et al.,[5] Taqi[6] Xing et al.,[20] and Rajpura et al.[21] Correlating the salivary sialic acid level with different histopathologic grades of squamous cell carcinoma, statistically, significant difference was found between control and moderately/poorly differentiated group and between well-differentiated and moderately/poorly differentiated group, which was in contrast with that of Shivashankara and Prabhu[4] and Sanjay et al.[10] There was no significant difference between control and well-differentiated squamous cell carcinoma, which may be due to the significant increase in release/shedding of sialic acids occurring at later phase in OSCC.[1] These contrasts in results may be due to the difference in sampling, i.e., number of patients with different histopathological grades of OSCC.
Increase in salivary sialic acid levels with histopathologic grades of carcinoma correlates with progression of cancer. It may be of prognostic value as its level increases with increasing grades of cancer. Salivary sialic acid level may not be used as a diagnostic marker as the control and well-differentiated squamous cell carcinoma group did not show any difference.
Statistically, significant difference was observed in the serum sialic acid level between control and moderately/poorly differentiated groups which contrasts the study by Joshi and Patil[1] and Taqi.[6] Serum sialic acid level was found to increase with progressive histopathologic grades of carcinoma.
Sialic acid levels in both saliva and serum of well-differentiated group were not elevated as in previous studies. It may be attributed to the early stage of our samples. The circulatory levels of serum total sialic acid increase with disease progression and with the tumor burden.[1]
We did not find any correlation between the salivary and serum sialic acid levels in control group, but a significant positive correlation was observed in OSCC group. This can be attributed to proximity of the tumor to the saliva. Serum sialic acid levels may be elevated due to the sialic acid-containing soluble proteins released as an inflammatory response from liver.[22] However, there is no statistically significant correlation between serum and salivary sialic acid level in control and well-differentiated squamous cell carcinoma group. There is observable correlation between serum and salivary sialic acid level in moderately/poorly differentiated squamous cell carcinoma group because of smaller sample size.
CONCLUSION
There is elevated serum and salivary sialic acid level in moderately/poorly differentiated squamous cell carcinoma without any significant change in well-differentiated squamous cell carcinoma. As the histopathological grade progresses, there is a marked increase in levels of sialic acid. There is a significant positive correlation between serum and salivary sialic acid levels in OSCC. Further research with larger sample size along with other grading and staging system may highlight its significance in OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Joshi M, Patil R. Estimation and comparative study of serum total sialic acid levels as tumor markers in oral cancer and precancer. J Cancer Res Ther. 2010;6:263–6. doi: 10.4103/0973-1482.73339. [DOI] [PubMed] [Google Scholar]
- 2.Raval GN, Parekh LJ, Patel DD, Jha FP, Sainger RN, Patel PS. Clinical usefulness of alterations in sialic acid, sialyl transferase and sialoproteins in breast cancer. Indian J Clin Biochem. 2004;19:60–71. doi: 10.1007/BF02894259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markopoulos AK, Michailidou EZ, Tzimagiorgis G. Salivary markers for oral cancer detection. Open Dent J. 2010;4:172–8. doi: 10.2174/1874210601004010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivashankara AR, Prabhu KM. Salivary total protein, sialic acid, lipid peroxidation and glutathione in oral squamous cell carcinoma. Biomed Res. 2011;22:355–9. [Google Scholar]
- 5.Raval GN, Patel DD, Parekh LJ, Patel JB, Shah MH, Patel PS. Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. Oral Dis. 2003;9:119–28. doi: 10.1034/j.1601-0825.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 6.Taqi SA. Clinical evaluation of total and lipid bound sialic acid levels in oral precancer and oral cancer. Indian J Med Paediatr Oncol. 2012;33:36–41. doi: 10.4103/0971-5851.96967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskaran K, Satish R, Santhosh V, Santha K, Manoharan S, Inmozhi R, et al. Status of sialic acid in patients with cervical intraepithelial neoplasia and cervical carcinoma. J Cell Tissue Res. 2010;10:2375–8. [Google Scholar]
- 8.Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Adv Cancer Res. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]
- 9.Shantaram M, Rao A, Aroor AR, Raja A, Rao S, Monteiro F. Assessment of total sialic acid and lipid-bound sialic acid in management of brain tumors. Ann Indian Acad Neurol. 2009;12:162–6. doi: 10.4103/0972-2327.56315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanjay PR, Hallikeri K, Shivashankara AR. Evaluation of salivary sialic acid, total protein, and total sugar in oral cancer: A preliminary report. Indian J Dent Res. 2008;19:288–91. doi: 10.4103/0970-9290.44529. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava A, Saigal S, Chalishazar M. Histopathological grading systems in oral squamous cell carcinoma: A review. J Int Oral Health. 2010;2:1–10. [Google Scholar]
- 12.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–5. [PubMed] [Google Scholar]
- 13.Navazesh M. Methods for collecting saliva. Ann NY Acad Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 14.Yao K, Ubuka T, Masuoka N, Kinuta M, Ikeda T. Direct determination of bound sialic acids in sialoglycoproteins by acidic ninhydrin reaction. Anal Biochem. 1989;179:332–5. doi: 10.1016/0003-2697(89)90138-3. [DOI] [PubMed] [Google Scholar]
- 15.Kurtul N, Cil MY, Paçaci SD. Serum total sialic acid levels in smokers and users of smokeless tobacco in form of oral powder (Maras powder) J Biomed Sci. 2005;12:559–63. doi: 10.1007/s11373-005-4563-x. [DOI] [PubMed] [Google Scholar]
- 16.Macbeth RA, Bekesi JG. Plasma glycoproteins in various disease states including carcinoma. Cancer Res. 1962;22:1170–6. [Google Scholar]
- 17.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 18.Heimer R, Meyer K. Studies on sialic acid of submaxillary mucoid. Proc Natl Acad Sci U S A. 1956;42:728–34. doi: 10.1073/pnas.42.10.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav A, Gahlaut V, Gahlaut PS. Serum total sialic acid as a marker for prognosis of chemotherapy in patients of acute leukemia. Res J Pharm Biol Chem Sci. 2011;2:220–5. [Google Scholar]
- 20.Xing RD, Chen RM, Wang ZS, Zhang YZ. Serum sialic acid levels in patients with oral and maxillofacial malignancy. J Oral Maxillofac Surg. 1991;49:843–7. doi: 10.1016/0278-2391(91)90013-c. [DOI] [PubMed] [Google Scholar]
- 21.Rajpura KB, Patel PS, Chawda JG, Shah RM. Clinical significance of total and lipid bound sialic acid levels in oral pre-cancerous conditions and oral cancer. J Oral Pathol Med. 2005;34:263–7. doi: 10.1111/j.1600-0714.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 22.Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Biochemical engineering of the N-acyl side chain of sialic acid: Biological implications. Glycobiology. 2001;11:11R–18. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]


