Abstract
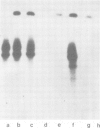
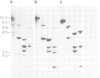
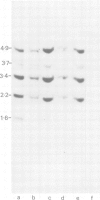
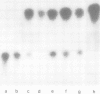
The dominant selectable gene, Ecogpt, has been introduced, by the calcium phosphate precipitation technique, into normal human fibroblasts, along with the SV40 early region genes. In one transfectant clone, integration of these sequences into human chromosome 17 was demonstrated by the construction of human-mouse somatic cell hybrids, selected for by growth in medium containing mycophenolic acid and xanthine. A whole cell hybrid, made between the human transfectant and a mouse L cell, was used as donor of the Ecogpt-carrying human chromosome 17 to 'tribrids' growing in suspension, made by whole cell fusion between a mouse thymoma cell line, and to microcell hybrids made with a mouse teratocarcinoma cell line. Two tribrids contained karyotypically normal human chromosomes 17 and a small number of other human chromosomes, while a third tribrid had a portion of the long arm of chromosome 17 translocated to mouse as its only human genetic material. Two independent microcell hybrids contained a normal chromosome 17 and no other human chromosome on a mouse teratocarcinoma background. These experiments demonstrate the ability to construct human-mouse somatic cell hybrids using a dominant selection system. By applying this approach it should be possible to select for a wide range of different human chromosomes in whole cell and microcell hybrids. In particular, transfer of single human chromosomes to mouse teratocarcinoma cells will allow examination of developmentally regulated human gene sequences after differentiation of such hybrids.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai Y., Sheer D., Hiorns L., Knowles R. W., Tunnacliffe A. A monoclonal antibody recognizing a cell surface antigen coded for by a gene on human chromosome 17. Ann Hum Genet. 1982 Oct;46(Pt 4):337–347. doi: 10.1111/j.1469-1809.1982.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Begovich A., Francke U. Karyotype evolution of the simian virus 40--transformed human cell line LNSV. Cytogenet Cell Genet. 1979;23(1-2):3–11. doi: 10.1159/000131296. [DOI] [PubMed] [Google Scholar]
- Bishop C. E., Lewis C. M., Festenstein H. Dominant allele-specific regulation of expression of H-2Kk gene products revealed by somatic cell hybridization. Somatic Cell Genet. 1982 Sep;8(5):623–634. doi: 10.1007/BF01542855. [DOI] [PubMed] [Google Scholar]
- Bobrow M., Cross J. Differential staining of human and mouse chromosomes in interspecific cell hybrids. Nature. 1974 Sep 6;251(5470):77–79. doi: 10.1038/251077a0. [DOI] [PubMed] [Google Scholar]
- Bucchini D., Lasserre C., Kunst F., Lovell-Badge R., Pictet R., Jami J. Stable transformation of mouse teratocarcinoma stem cells with the dominant selective marker Eco.gpt and retention of their developmental potentialities. EMBO J. 1983;2(2):229–232. doi: 10.1002/j.1460-2075.1983.tb01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo M. S., Cameron I. R., Rogers M. E. Tandem integration of complete and defective SV40 genomes in mouse-human somatic cell hybrids. Cell. 1978 Dec;15(4):1411–1426. doi: 10.1016/0092-8674(78)90065-x. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Lomakka G., Zech L. The 24 fluorescence patterns of the human metaphase chromosomes - distinguishing characters and variability. Hereditas. 1972;67(1):89–102. doi: 10.1111/j.1601-5223.1971.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Assignment of the integration site for simian virus 40 to chromosome 17 in GM54VA, a human cell line transformed by simian virus 40. Proc Natl Acad Sci U S A. 1977 Jan;74(1):315–318. doi: 10.1073/pnas.74.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Girardi A. J., Koprowski H. Assignment of the T-antigen gene of simian virus 40 to human chromosome C-7. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3617–3620. doi: 10.1073/pnas.70.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Dubbs D. R., Kit S. Chromosomal site(s) of integration of Herpes simplex virus type 2 thymidine kinase gene in biochemically transformed human cells. Int J Cancer. 1977 Aug 15;20(2):256–267. doi: 10.1002/ijc.2910200214. [DOI] [PubMed] [Google Scholar]
- Fournier R. E. A general high-efficiency procedure for production of microcell hybrids. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6349–6353. doi: 10.1073/pnas.78.10.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Ruddle F. H. Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):319–323. doi: 10.1073/pnas.74.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Trowsdale J., Chambers S., Solomon E. Introduction of a human X-6 translocation chromosome into a mouse teratocarcinoma: investigation of control of HLA-A, B, C expression. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1190–1194. doi: 10.1073/pnas.79.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Levy R., Povey S., McMichael A. A human X-linked antigen defined by a monoclonal antibody. Somatic Cell Genet. 1980 Nov;6(6):777–787. doi: 10.1007/BF01538976. [DOI] [PubMed] [Google Scholar]
- Goodfellow P., Banting G., Sheer D., Ropers H. H., Caine A., Ferguson-Smith M. A., Povey S., Voss R. Genetic evidence that a Y-linked gene in man is homologous to a gene on the X chromosome. Nature. 1983 Mar 24;302(5906):346–349. doi: 10.1038/302346a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Henderson N. S. Isozymes and genetic control of NADP-malate dehydrogenase in mice. Arch Biochem Biophys. 1966 Oct;117(1):28–33. doi: 10.1016/0003-9861(66)90121-4. [DOI] [PubMed] [Google Scholar]
- Huerre C., Junien C., Weil D., Chu M. L., Morabito M., Van Cong N., Myers J. C., Foubert C., Gross M. S., Prockop D. J. Human type I procollagen genes are located on different chromosomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6627–6630. doi: 10.1073/pnas.79.21.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. P., Kucherlapati R. Localization and organization of integrated simian virus 40 sequences in a human cell line. Virology. 1980 Aug;105(1):196–204. doi: 10.1016/0042-6822(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Kucherlapati R. S., Baker R. M., Ruddle F. H. Ouabain as a selective agent in the isolation of somatic cell hybrids. Birth Defects Orig Artic Ser. 1975;11(3):192–193. [PubMed] [Google Scholar]
- Kucherlapati R., Hwang S. P., Shimizu N., McDougall J. K., Botchan M. R. Another chromosomal assignment for a simian virus 40 integration site in human cells. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4460–4464. doi: 10.1073/pnas.75.9.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Long C., Green H. A new reduced human-mouse somatic cell hybrid containing the human gene for adenine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1971 Jan;68(1):82–86. doi: 10.1073/pnas.68.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay M. A., Wilson D. E., Harrison B., Povey S. Association of herpes simplex thymidine kinase gene with chromosome No. 18 in transformed human cells. J Natl Cancer Inst. 1980 Feb;64(2):241–248. doi: 10.1093/jnci/64.2.241. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- McNeill C. A., Brown R. L. Genetic manipulation by means of microcell-mediated transfer of normal human chromosomes into recipient mouse cells. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5394–5398. doi: 10.1073/pnas.77.9.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Schlegel R. A. Phytohemagglutinin enhancement of cell fusion reduces polyethylene glycol cytotoxicity. Exp Cell Res. 1979 May;120(2):417–421. doi: 10.1016/0014-4827(79)90403-8. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Ramsey G. A., Krenitsky T. A., Elion G. B. Guanine phosphoribosyltransferase from Escherichia coli, specificity and properties. Biochemistry. 1972 Dec 5;11(25):4723–4731. doi: 10.1021/bi00775a014. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabholz M., Miggiano V., Bodmer W. Genetic analysis with human--mouse somatic cell hybrids. Nature. 1969 Jul 26;223(5204):358–363. doi: 10.1038/223358a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975 Oct;1(4):397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr Human cell transformation by simian virus 40--a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- Segal S., Levine A. J., Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979 Jul 26;280(5720):335–338. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Smiley J. R., Steege D. A., Juricek D. K., Summers W. P., Ruddle F. H. A herpes simplex virus 1 integration site in the mouse genome defined by somatic cell genetic analysis. Cell. 1978 Oct;15(2):455–468. doi: 10.1016/0092-8674(78)90015-6. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T., Croce C. M. Selective induction of murine oncornavirus gene expression in somatic cell hybrids between mouse peritoneal macrophages and SV-40-transformed human cells. Virology. 1976 Apr;70(2):545–549. doi: 10.1016/0042-6822(76)90296-8. [DOI] [PubMed] [Google Scholar]
- Sundar Raj C. V., Church R. L., Klobutcher L. A., Ruddle F. H. Genetics of the connective tissue proteins: assignment of the gene for human type I procollagen to chromosome 17 by analysis of cell hybrids and microcell hybrids. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4444–4448. doi: 10.1073/pnas.74.10.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Mintz B. Transfer of nonselectable genes into mouse teratocarcinoma cells and transcription of the transferred human beta-globin gene. Mol Cell Biol. 1982 Feb;2(2):190–198. doi: 10.1128/mcb.2.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Differentiation antigens of the lymphocyte cell surface. Contemp Top Mol Immunol. 1977;6:83–116. doi: 10.1007/978-1-4684-2841-4_3. [DOI] [PubMed] [Google Scholar]