Abstract
Background:
Infectious diseases have always been an important health issue in human communities. In the recent years, much research has been conducted on antimicrobial effects of nature-based compounds because of increased prevalence of antibiotic resistance. The present study was conducted to investigate synergistic effect of Carum copticum and Mentha piperita essential oils with ciprofloxacin, vancomycin, and gentamicin on Gram-negative and Gram-positive bacteria.
Materials and Methods:
In this experimental study, the synergistic effects of C. copticum and M. piperita essential oils with antibiotics on Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus epidermidis (ATCC 14990), and Listeria monocytogenes (ATCC 7644) were studied according to broth microdilution and the MIC and fractional inhibitory concentration (FIC) of these two essential oils determined.
Results:
C. copticum essential oil at 30 μg/ml could inhibit S. aureus, and in combination with vancomycin, decreased MIC from 0.5 to 0.12 μg/ml. Moreover, the FIC was derived 0.24 μg/ml which represents a potent synergistic effect with vancomycin against S. aureus growth. C. copticum essential oil alone or combined with other antibiotics is effective in treating bacterial infections.
Conclusions:
In addition, C. copticum essential oil can strengthen the activities of certain antibiotics, which makes it possible to use this essential oil, especially in drug resistance or to lower dosage or toxicity of the drugs.
Keywords: Antimicrobial effect, essential oil, pathogenic bacteria, plant, synergism
INTRODUCTION
Microbial diseases remain an important health issue in human communities which has been targeted by the efforts of many researchers in medical, health, and laboratory areas. Despite development of different antibiotics, the problem of microbial resistance to the antibiotics highlights the necessity of seeking out new antimicrobial compounds.[1] According to evidence on emergence of resistance in microorganisms to antibiotics, use of antimicrobial plant-based compounds has further increased in the recent years.[1]
Despite confirmed antimicrobial effects of medicinal plants and nature-based compounds, use of them to treat infections has not become common and physicians continue to prescribe chemical antibiotics. Many medicinal plants are being used to treat microbial diseases.[2,3,4,5]
Carum copticum is from family Apiaceae. C. copticum is originally from Asia and spontaneously occurs or is cultivated in India, Iran, and Egypt. This plant is annual and its seeds are usable.[6,7,8] Phytochemical studies have demonstrated that over 85% of the compounds of C. copticum essential oil are thymol, gamma-terpinene, and paracymene. Thymol can exert hypotensive effects and be used to treat skin diseases such as acne, psoriasis, and dermatitis in combination with other phenolic compounds.[9] Gamma-terpinene is used in perfume industry and paracymene is used in perfume and pharmaceutical industries as they can help different drugs be absorbed through skin pores and have antibacterial and antifungal effects. C. copticum fruit extract can be used to heal wounds.[10] Besides that, C. copticum fruit has been reported to exert anti-nausea and antifungal effects.[10]
Peppermint, botanically called Mentha piperita L., is from family Lamiaceae. M. piperita is a medicinal and fragrant plant whose essential oil has many pharmaceutical, food, and cosmetic uses. M. piperita is a herbaceous and perennial plant and has creeping and underground stems, the stem is rectangular and purplish red, the leaves are elliptical, and positioned as crossed on the stem. The flowers are purple and the fruit is red and capsule-shaped, and has a nongerminative seed.[11] The compounds of M. piperita essential oil have antioxidant, fungicidal, and insecticidal effects.[12,13,14] The antimicrobial effects of C. copticum on Candida albicans, Escherichia coli, and Staphylococcus aureus have been confirmed.[15] In addition, it has been demonstrated that M. piperita essential oil causes elimination of any Salmonella species[16] and exerts inhibitory effects on C. albicans.[17] Analysis of M. piperita essential oil has indicated that this plant contains ethyl ether, acetic acid, isomenthol, polygon, neomenthol, menthol, piperitone, and neoisomenthol.[18] However, a more important argument is concerned with reduction in the side effects and toxic effects of antibiotics in patients and antibiotic resistance.
Therefore, in this experimental study, the synergistic effects of C. copticum and M. piperita essential oils with antibiotics on S. aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), E. coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), Staphylococcus epidermidis (ATCC 14990), and Listeria monocytogenes (ATCC 7644) were studied according to broth microdilution.
MATERIALS AND METHODS
Preparation of medicinal plants and antibiotics
C. copticum and M. piperita were provided from Darab, Fars province. Table 1 shows botanical characteristics of these two plants.
Table 1.
Botanical characteristics of Carum copticum and Mentha Piperita

C. copticum and M. piperita essential oils were purchased from a pharmaceutical company (Saghar Darab Fars) and assessed by broth microdilution.
Preparation of essential oils
The essential oil initial concentration was 850 μg/1000 ml. The resulting substance was first dissolved in 0.5 ml hexane and diluted with phosphate buffered saline at 1:5 or 1:20 rate. Before tests of minimum bacteriostatic concentration (MIC) and minimum bactericidal concentration (MBC), the essential oils were sterilized by passing through a 0.22-μm filter (Jet Bio-Filtration Co., China). The densities of the essential oils were determined manually through calculation of the essential oil's mass and volume as well as by densitometer (the mean pure density of the essential oil was determined 850 μg/ml). Different purities of a number of antibiotics (vancomycin, gentamicin, and ciprofloxacin [Sigma, USA]) were also assessed both in combination with the essential oils and separately as controls and the synergistic effects were investigated by determination of the MBC and MIC. Each experiment was conducted in triplicate and the mean values were included in data analysis.
Preparation of the studied bacteria
Broth microdilution
To prepare microbial suspension, 24-h culture was prepared from each bacterium. The bacteria were inoculated on nutrient agar culture medium and then incubated for 24 h. To do this, the 24-h culture of the bacteria on Mueller-Hinton agar medium was introduced into normal saline and an opacity equal to 0.5 McFarland developed with an approximately 1.5 × 108 CFU/ml bacteria in the suspension.[19]
Determining MIC
In this study, the MIC was tested according to the method recommended by the National Committee for Clinical Laboratory Standards in a 96-well sterile plate according to the broth microdilution method. First, 100 μl of Mueller-Hinton broth culture medium (Merck, Germany) was introduced into 96-well microplate. Then, 100 μl of the essential oil was added to the first wall in each row and dilution was conducted in 2nd–9th wells. The dilution in each well was determined and registered in view of the essential oil's volume and density. Finally, 100 μl of the microbial suspension was added to all wells and their opacity was investigated by tray reading stand plate base after 24-h incubation at 37°C for 24 h. The concentration of the last (most diluted) well with no opacity was considered MIC.
Determining minimum bactericidal concentration
To investigate the MBC, all wells without opacity were cultured on Mueller-Hinton broth culture medium, and after 24-h incubation, the lowest concentration of the essential oil in which the bacteria did not grow (decrease in CFU by 99.9% lower than the control) was reported to be the MBC.
Determining synergistic effect
In determining the MIC and the MBC, the concentration of the essential oil or antibiotic was decided to be 0.25–25 μg/ml according to the purity percentage of the antibiotic and then dilution serially conducted. Afterward, 50 μl of each dilution was poured into the next wall (perpendicular to the direction of serial dilution) such that each well contained 50 μl of the essential oil and 50 μl of the antibiotic instead of 100 μl of the essential oil and 100 μl of the antibiotic, respectively.
By this way, in the 96-well plate, the essential oil and antibiotics at different dilutions were combined with each other. After addition of 100 μl of the microbial suspension as described above, the dilutions of the MIC and MBC of the antibiotics, essential oil, and their combination were determined. Then, the fractional inhibitory concentration (FIC) was computed as follows:
The combination's MIC was divided by the antibiotic's MIC. If the result was lower than 0.5, the two compounds had synergistic effect, if it was 0.5–1, the effect was additive, if it was 1–4, combination of them was considered to have no effect, and if it was ≥4, the effect of the two compounds was considered to be antagonist.[20]
Data analysis
The findings on antibiotics were registered in the tables of the MIC, MBC, and FIC. The experiments were conducted in triplicate and the mean values were included in the data analysis.
RESULTS
In this study, the synergistic effects of C. copticum and M. piperita essential oils on a number of pathogenic bacteria were investigated. The method of the experiments was broth microdilution, the experiments were conducted in triplicate, and the mean values of the three experiments' results are shown in Tables 2–11.
Table 2.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Carum copticum and combination of Carum copticum and vancomycin for Enterococcus faecalis

Table 11.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Mentha piperita essential oil and combination of Mentha piperita essential oil and vancomycin for Staphylococcus aureus
In this study, the synergistic effect of C. copticum with vancomycin on E. faecalis was investigated and the FIC was derived three that represents lack of effect of the combination of these two compounds [Table 2].
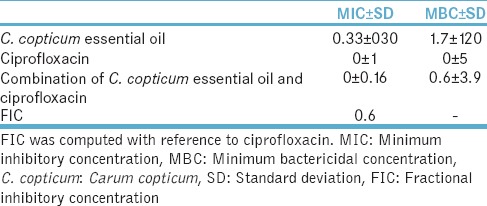
The results demonstrated that 30 μg/ml of C. copticum could inhibit E. coli and combined with ciprofloxacin could decrease the MBC from 5 to 3.9 μg/ml and the MIC from 3.6 to 0.6 μg/ml, and the FIC was 0.6 that represents lack of effect of the combination of these two compounds on E. coli growth [Table 3].
Table 3.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Carum copticum essential oil and combination of Carum copticum essential oil and ciprofloxacin for Escherichia coli
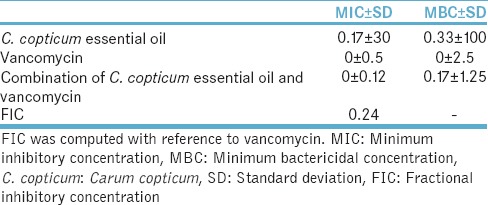
Moreover, the results demonstrated that 30 μg/ml of C. copticum could inhibit S. aureus growth and combined with vancomycin could decrease the MIC from 0.5 to 0.12 μg/ml, and it displayed a potent inhibitory effect on S. aureus growth such that the FIC was derived 0.24 that is very high [Table 4].
Table 4.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Carum copticum essential oil and combination of Carum copticum essential oil and vancomycin for Staphylococcus aureus
The results demonstrated that 68 μg/ml of C. copticum could inhibit S. epidermidis growth and combined with ciprofloxacin could decrease the MBC from 4 to 1.6 μg/ml that can be considered a significant effect.
In addition, the synergistic effect of C. copticum with ciprofloxacin on S. epidermidis growth was studied and found to cause the MIC of 4 μg/ml to reach 16 μg/ml, which represents no synergistic effect [Table 5].
Table 5.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Carum copticum essential oil and combination of Carum copticum essential oil and ciprofloxacin for Staphylococcus epidermidis

The results showed that 544 μg/ml of C. copticum essential oil displayed effect on P. aeruginosa growth and combined with gentamicin exerted no effect [Table 6].
Table 6.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Carum copticum essential oil and combination of Carum copticum essential oil and gentamicin for Pseudomonas aeruginosa

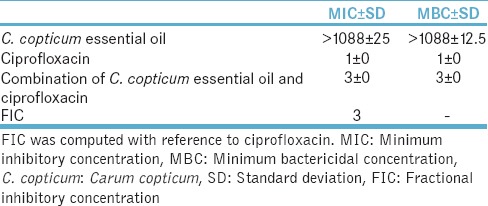
C. copticum essential oil at 1088 μg/ml caused inhibition of L. monocytogenes and combined with ciprofloxacin had FIC of 3 that represents lack of effect on L. monocytogenes growth [Table 7].
Table 7.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Carum copticum essential oil and combination of Carum copticum essential oil and ciprofloxacin for Listeria monocytogenes
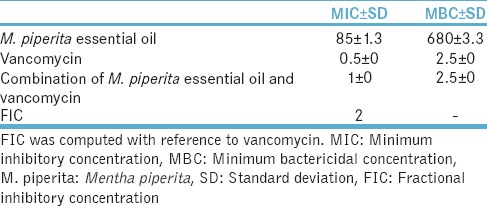
M. piperita essential oil at 4200 μg/ml caused inhibition of E. faecalis and combined with vancomycin had FIC of 2 that represents lack of effect on E. faecalis growth [Table 8].
Table 8.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Mentha piperita essential oil and combination of Mentha piperita essential oil and vancomycin for Enterococcus faecalis

The results demonstrated that 128 μg/ml of M. piperita could inhibit E. coli growth and combined with ciprofloxacin caused increase in the MIC from 1 to 3 μg/ml. The combination FIC for E. coli was three that represents lack of effect on E. coli growth [Table 9].
Table 9.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Mentha piperita essential oil and combination of Mentha piperita essential oil and ciprofloxacin for Escherichia coli

The results demonstrated that 212 μg/ml of M. piperita could inhibit S. epidermidis growth and combined with ciprofloxacin displayed FIC of 3 that represents lack of effect on S. epidermidis growth [Table 10].
Table 10.
Mean (standard deviation) minimum inhibitory concentration and minimum bactericidal concentration (μg/ml) of Mentha piperita essential oil and combination of Mentha piperita essential oil and ciprofloxacin for Staphylococcus epidermidis

The results demonstrated that 85 μg/ml of M. piperita could inhibit S. aureus growth and combined with ciprofloxacin displayed FIC of 2 that represents lack of effect on S. aureus growth [Table 11].
Unfortunately, it was not possible to investigate such effects on other bacteria, especially resistant ones. These effects should be investigated on a greater number of bacteria especially Gram-negative ones so that the findings may be inclusive enough to be used in clinical trials. Besides that, the synergistic effect should be investigated with novel antibiotics suitable for each bacterium such as third- and fourth-generation cephalosporins. However, the current study is considered a preliminary work.
DISCUSSION
The antibacterial properties of C. copticum and M. piperita have already been confirmed. According to the experiments' results in the current study, C. copticum and M. piperita have antibacterial properties such that M. piperita essential oil at 85 μg/ml dilution could inhibit S. aureus growth. C. copticum at 30 and 68 μg/ml could inhibit the growth of S. aureus and S. epidermidis, respectively. Briefly, the antibacterial effects of these two essential oils were more potent on S. aureus, and the antibacterial effect of C. copticum essential oil was more potent than that of M. piperita on the same bacteria.
In this study, the synergistic effects of C. copticum and M. piperita essential oils with antibiotics on a number of bacteria were investigated. The findings demonstrated that C. copticum essential oil had a potent antibacterial effect and displayed a relatively potent synergistic effect with vancomycin (FIC = 0.24). M. piperita essential oil displayed no synergistic effect with the studied antibiotics on S. epidermidis, S. aureus, E. coli, and E. faecalis. A study on the synergistic effect of some plant-based essential oils with antibiotics demonstrated that M. piperita essential oil had no synergistic effect with ampicillin, erythromycin, and gentamicin (FIC = 1 for all).[21] The current study demonstrated that M. piperita exerted no synergistic effect with ciprofloxacin and vancomycin (FIC: 2–3). In our study, an approximately similar FIC was derived for C. copticum essential oil synergistic effect with vancomycin on S. aureus growth.
The findings of an experimental study demonstrated that Cuminum cyminum exerted synergistic effect with gentamicin on food-borne microorganisms according to disk diffusion method.[22] In the present study, C. copticum synergistic effect with gentamicin on P. aeruginosa was investigated by broth microdilution and no effect was seen with FIC of 2. Fadli et al.'s study on synergistic effects of 80 compounds of Thymus vulgaris essential oil with common antibiotics concluded that 71% of this plant's compounds displayed synergistic effect, 20% displayed relatively synergistic effect, and 9% displayed no effect on the studied bacteria.
In that study, T. vulgaris essential oil synergistic effect with ciprofloxacin on E. coli was found to have FIC of 0.12. In the current experimental study, the synergistic effects of C. copticum essential oil and M. piperita essential oil with ciprofloxacin on E. coli were found to have FIC of 0.6 and 3, respectively, which is in disagreement with Fadlie et al.'s study. This inconsistency in the findings can be due to difference in the percentages the chemical compounds among the essential oils.[23] In addition, Fadlie et al. reported that the synergistic effect of T. vulgaris essential oil with ciprofloxacin on S. aureus had an FIC of 0.26, but in the current study, M. piperita essential oil was found to have no synergistic effect with ciprofloxacin. A study investigated the synergistic effect of Chrysanthemum indicum with ampicillin and gentamicin on the growth of some species of bacteria using broth microdilution and concluded that C. copticum essential oil combined with ampicillin could decrease MIC of E. coli, S. aureus, and S. epidermidis by 1:2–1:6 but did not display synergistic effect (FIC of 0.5–1).[24]
In this study, C. copticum essential oil exerted synergistic effect with gentamicin on S. aureus such that the MIC decreased from 3.2 to 2.0 μg/ml and FIC was 0.25 that represented their synergistic effect. In the current study, C. copticum essential oil exerted synergistic effect with vancomycin on S. aureus, which can be due to S. aureus susceptibility to the compounds in the plant-based essential oils that needs further research.
CONCLUSIONS
C. copticum can exert a potent antibacterial effect that can cause a significant synergistic effect if accompanied by the antibiotics. In this study, it was demonstrated that C. copticum essential oil exerted synergistic effect with vancomycin. It is expected that these plant-based essential oils alongside antibiotics be incorporated into health-care system through further studies so that their useful effects can be used to fight drug resistance or to lower dosage or toxicity of the drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol. 2000;70:343–9. doi: 10.1016/s0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 2.Taherikalani M, Hassanzadazar H, Bahmani M, Baharvand-Ahmadi B, Rafieian-Kopaei M. Staphylococcus phytotherapy: An overview on the most important Iranian native medicinal plants effective on Staphylococcus aureus. J Chem Pharm Sci. 2016;9:16–23. [Google Scholar]
- 3.Rafieian-Kopaei M, Saki K, Bahmani M, Ghafourian S, Sadeghifard N, Taherikalani M. Listeriosis phytotherapy: A review study on the effectiveness of Iranian medicinal plants in treatment of listeriosis. J Evid Based Complementary Altern Med. 2015:pii: 2156587215621460. doi: 10.1177/2156587215621460. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsaei P, Bahmani M, Naghdi N, Asadi-Samani M, Rafieian-Kopaei MM, Boroujeni S. Shigellosis phytotherapy: A review of the most important native medicinal plants in Iran effective on Shigella. J Chem Pharm Sci. 2016;8:249–55. [Google Scholar]
- 5.Rafieian-Kopaei M, Bahmani M, Abaszadeh A, Hassanzadazar H, Soroush S, Kazemzadeh F. Salmonellosis phytotherapy: A review on Iranian most important medicinal plants affecting on Salmonella. J Chem Pharm Sci. 2016;9:1313–21. [Google Scholar]
- 6.Boskabady MH, Jandaghi P, Kiani S, Hasanzadeh L. Antitussive effect of Carum copticum in guinea pigs. J Ethnopharmacol. 2005;97:79–82. doi: 10.1016/j.jep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol. 2005;98:127–35. doi: 10.1016/j.jep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Gersbach PV, Reddy N. Non-invasive localization of thymol accumulation in Carum copticum (Apiaceae) fruits by chemical shift selective magnetic resonance imaging. Ann Bot. 2002;90:253–7. doi: 10.1093/aob/mcf179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava M, Saxena A, Baby P. GC-MS investigation and anti microbial activity or the essential oil of Carum copticum. Acta Alimentaria J. 1999;28:291–5. [Google Scholar]
- 10.Zargari A. Medicinal Plants. 2nd ed. Tehran: Tehran Press; 1993. pp. 30–62. [Google Scholar]
- 11.Foster S. Peppermint: Mentha piperita. American Botanical Council-Botanical Series, 1996;306:3–8. [Google Scholar]
- 12.Bouchra C, Achouri M, Idrissi Hassani LM, Hmamouchi M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J Ethnopharmacol. 2003;89:165–9. doi: 10.1016/s0378-8741(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 13.Pavela R. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia. 2005;76:691–6. doi: 10.1016/j.fitote.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry. 2006;67:1249–55. doi: 10.1016/j.phytochem.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Aridogan BC, Baydar H, Kaya S, Demirci M, Ozbasar D, Mumcu E. Antimicrobial activity and chemical composition of some essential oils. Arch Pharm Res. 2002;25:860–4. doi: 10.1007/BF02977005. [DOI] [PubMed] [Google Scholar]
- 16.Tassou CC, Drosinos EH, Nychas GJ. Effects of essential oil from mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in model food systems at 4° and 10°C. J Appl Microbiol. 1995;78:593–600. doi: 10.1111/j.1365-2672.1995.tb03104.x. [DOI] [PubMed] [Google Scholar]
- 17.Ezzat SM. In vitro inhibition of Candida albicans growth by plant extracts and essentialoils. World J Microbiol Biotechnol. 2001;39:757–9. [Google Scholar]
- 18.Lawrence BM. Composition of three oils of M. pulegium produced from plant grown in North Carolina. Perf Flav. 1998;76:691–6. [Google Scholar]
- 19.Khanmohammadi H, Arab V, Rezaeian KH, Talei GH, Pass M, Shabani N. Diaminomaleonitrile-based azo receptors: Synthesis, DFT studies and their antibacterial activities. J Mol Struct. 2017;1129:169–78. [Google Scholar]
- 20.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15:639–52. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Schelz Z, Molnar J, Hohmann J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia. 2006;77:279–85. doi: 10.1016/j.fitote.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Rosato A, Piarulli M, Corbo F, Muraglia M, Carone A, Vitali ME, et al. In vitro synergistic antibacterial action of certain combinations of gentamicin and essential oils. Curr Med Chem. 2010;17:3289–95. doi: 10.2174/092986710792231996. [DOI] [PubMed] [Google Scholar]
- 23.Daneshmandi S, Soleymani N, Satari M, Pourfathollah E. Pharmaceutical synergistic action and antibacterial effects of Corum oil. Aram Uni Med Sci J. 2010;13:72–6. [Google Scholar]
- 24.Fadli M, Saad A, Sayadi S, Chevalier J, Mezrioui NE, Pagès JM, et al. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection – Bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19:464–71. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]


