Abstract
Purpose of Review
Spontaneous lymphoma in pet dogs is increasingly recognized as an ideal model for studying the disease in humans and for developing new targeted therapeutics for patients. Increasing interest by funding agencies, the private sector, and multidisciplinary academic collaborations between different disciplines and sectors now enable large knowledge gaps to be addressed and provide additional proof-of-concept examples to showcase the significance of the canine model.
Recent Findings
This review addresses the rationale for a canine lymphoma model including the valuable role it can play in drug development, serving as a link between mouse xenograft models and human clinical trials and the infrastructure that is now in place to facilitate these studies. Research in this field has focused on filling in the gaps in order to make the canine lymphoma model more robust. These advances have included work on biomarkers, detection of minimal residual disease, expansion of genomic and proteomic data, and immunotherapy.
Summary
Incorporating pet dogs into the drug development pipeline can improve the efficiency and predictability of preclinical models and decrease the time and cost required for a therapeutic target to be translated into clinical benefit.
Keywords: Canine model, lymphoma, immunotherapy, comparative oncology, dog
Introduction
Naturally occurring cancer in pet dogs has in recent years garnered the attention of researchers as a potentially representative and predictive model for human cancer. Canine lymphoma in particular shares many of the characteristics of non-Hodgkin lymphoma (NHL) in people, having a similar clinical presentation, molecular and immunophenotypic composition, diagnostic and therapeutic protocols, and treatment response (Figure 1). Spontaneous lymphoma in dogs is now being advocated as the link between mouse models and human clinical trials in testing new lymphoma therapies, with the potential to alleviate some of the bottlenecks in the traditional drug development pipeline (Figure 2). In the past year, growing efforts have been made to advance the canine model through investigations of the applicability of diagnostic and prognostic human biomarkers to dogs with lymphoma, expansion of canine genomic, proteomic, and other –omic data, and developing additional canine lymphoid cell line resources. This past year has also seen an expansion of immunotherapy research and the preclinical testing of novel targeted agents in canine cells and patients, highlighting the particularly exciting opportunities of the canine model for studying these agents. This review discusses the rationale for a canine model and the advances that are making canine lymphoma a more robust and more widely accepted model for the human disease.
Figure 1.
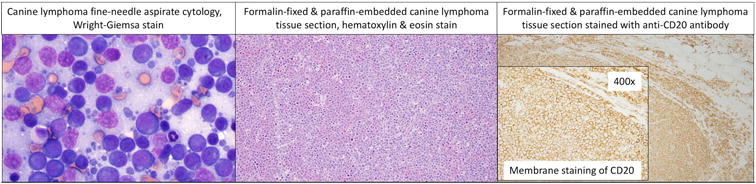
Canine diffuse large B-cell lymphoma (DLBCL) is cytologically and histologically similar to human DLBCL. a) Wright-Giemsa stained cytology from a fine-needle aspirate of an enlarged lymph node in a canine patient. b) Hematoxylin and eosin stained histologic section from a canine lymph node biopsy. a) & b) The main cell population in these two different cases are large lymphocytes greater than twice the size of a RBC. c) Same case as shown in b) with diffuse CD20 staining indicating a B-cell lineage. Images courtesy of Drs. Tracy Stokol and Jimmy Tran.
Figure 2.
Advantages of the spontaneous canine lymphoma model.
Rationale for a canine model of lymphoma
Currently it takes 16 years for a new drug to come to market at a $2 billion cost, and only 1 out of 10,000 compounds become FDA approved [1]. Even among those oncology drugs that are effective in mice, only 11% are eventually approved for use in humans [2]. A significant cause of this high failure rate is likely the reliance on rodents as preclinical models, when they frequently do not reflect the tumor biology and pathophysiology occurring in human patients [3]. Mouse models used in drug development have compromised immune systems, decreased genetic heterogeneity, and rarely develop naturally occurring lymphoma [4]. These mice lack complex genetic and gene-environment effects and their immunoincompetence ignores a central mechanism of cancer risk and development. In contrast, pet dogs naturally develop lymphoma at a higher incidence rate than humans (13-114 per 100,000 individuals compared to 20 per 100,000 in humans) [5] and have clinical presentations and diagnostic features comparable to that in people [6]. Diffuse large B-cell lymphoma (DLBCL) is the most common subtype in humans and dogs, accounting for ∼70% of canine B-cell lymphomas [5]. Dogs are also more genetically similar to humans, have increased genetic variation and intact immune systems, and are exposed to the same environmental risk factors as their owners, perhaps being able to serve as sentinels for these environmental hazards [4,7]. State-of-the-art veterinary care is readily available and pet owners are usually eager to enroll dogs in clinical trials and maintain high compliance due to limited availability of therapies and expense of therapy off-study [2]. Minimally invasive procedures can be routinely carried out and since dogs typically present with palpable lymphadenopathy, serial biopsies are feasible (Figure 3) [8]. Although dogs require larger drug doses compared to mice, which incurs higher costs, they model the pharmacokinetic and toxicity properties of a new drug more readily. The shorter lifespan of dogs and the faster progression of lymphoma in this species in comparison to humans enables timely assessments of novel cancer treatments [4] and enables chemoprevention studies. By using a spontaneous canine lymphoma model, the time and cost required for a therapeutic target to be translated into clinical benefit can be reduced while the predictability of preclinical models can be increased.
Figure 3.
Similar to human medicine, canine patients like the one shown here presenting for lymphadenopathy at a veterinary hospital will undergo a complete work-up including a physical exam, complete blood counts and chemistry panels, imaging, fine-needle aspiration for cytology and flow cytometry, lymph node and bone marrow biopsy, and IHC. Treatment protocols are then selected based on clinical findings.
A sign that the canine lymphoma model is becoming more mainstream has been the development of much needed clinical trial infrastructure over the last decade. National organizations, such as the Veterinary Cancer Society, CTSA One Health Alliance (COHA), and the Veterinary Cooperative Oncology Group, bring together expertise for the development and standardization of clinical trials. The Comparative Oncology Program (COP) of the National Cancer Institute (NCI) has established a Comparative Oncology Trials Consortium (COTC) in order to guide rigorously controlled preclinical trials of new therapies intended for human patients [4]. A national canine cancer biospecimen repository by the Canine Comparative Oncology and Genomics Consortium (CCOGC) was established in 2007 as a forum for sharing resources and has distributed high quality samples to dozens of researchers (Mazcko C, personal communication). In 2015 a workshop hosted by the US National Academy of Medicine (formerly the Institute of Medicine) brought veterinarians, physicians, researchers, pharmaceutical representatives, granting agencies, and government regulators together to discuss how oncology trials in dogs can better contribute to human health [8,9**]. The workshop sparked new collaborations and addressed a major roadblock- the concern that a drug that shows unexpected toxicity or lack of efficacy in the dog might negatively affect its approval for use in humans. Reassurances by FDA officials will hopefully alleviate pharmaceutical concerns, as will more successfully completed trials that augment, rather than hinder, human therapeutic development. The American Veterinary Medical Association (AVMA) also launched a database of all open clinical trials in veterinary species this past year (ebusiness.avma.org/aahsd/study_search.aspx) as a resource for researchers recruiting participants in clinical studies and for veterinarians and pet owners searching for treatment options. This database is similar to that available for human clinical trials (ClinicalTrials.gov) and intends to include studies occurring worldwide.
Funding agencies are now starting to take notice of the potential of canine cancer models. Until recently, researchers using a spontaneous canine lymphoma model have depended on funding from their own institutions or from non-profit, animal health focused foundations such as the Morris Animal Foundation and the American Kennel Club (AKC) Canine Health Foundation. Historically, the NIH has provided limited funding for animal cancer models that haven't been developed as thoroughly as rodent models [1]. This appears to be changing, however, as the NIH in April 2016 announced funding for collaborations between veterinarians, physicians, and scientists to arrive at a better understanding of the molecular mechanisms of cancer in dogs [1]. At the time of preparation of this manuscript, there were two open Requests for Application (RFA) aiming to fund canine immunotherapy studies and trials [10]. Dogs make ideal models for developing immunotherapies as they have intact immune systems that have been antigenically challenged, in contrast to mouse models. Other traditionally human-focused cancer funding foundations such as the American Cancer Society (ACS) and the Leukemia and Lymphoma Society (LLS) are also now considering grants that include the canine cancer model for funding (Richards KL, McCleary-Wheeler AL, personal experience). Since few dogs are cured with current chemotherapy regimens, dogs offer an opportunity to bring new treatments to the frontline treatment setting, using novel agents not just in relapsed cases, which can be the most challenging to treat. They are the only species in which one can use a “species in kind” approach where clinical trials can be conducted with the knowledge of pharmacokinetics and toxicities evaluated in research animals of the same species [4]. Given its breed structure and increasingly well-defined genetics [11*], the canine lymphoma model is also well-suited for developing precision medicine approaches in which clinical and molecular data from the patient are analyzed to tailor treatment targeting different pathways specifically for that individual [3].
Recent molecular advances
Researchers have attempted to fill in the knowledge gaps in comparative oncology and to develop the reagents and tools necessary for expanding the use of the canine lymphoma model. Here, we focus on a select group of notable studies from this past year.
Applying human findings to canine lymphoma
Various proteins that have been previously identified as potential prognostic biomarkers for human lymphoma are now being studied in dogs. Two of the most important biomarkers in human DLBCL are BCL2 and MYC, with double expressors accounting for ∼35% of all DLBCL patients [12]. This double-expressor lymphoma (DEL) subset has a significantly worse outcome, and is being targeted therapeutically in ongoing clinical trials, as these patients are often not cured by standard R-CHOP chemotherapy. When canine DLBCL was assessed for BCL2 and MYC, all patients were found to have high expression of both proteins using human scoring cutoffs [13]. When these samples were separated into “high” and “low” groups, as defined by expression above or below the median within the group, there was no association with clinical outcome. Therefore, combined BCL2 and MYC expression appears to be uniformly high in canine lymphoma, and not prognostic of outcome.
The expression of CD25, the alpha subunit of the interleukin (IL-2) receptor, has been previously reported in various subtypes of human lymphoid tumors, and CD25-positivity was associated with a poorer prognosis [14]. Mizutani et al evaluated CD25 expression in canine lymphomas using immunohistochemistry (IHC) and flow cytometry and found, similar to the human work, that CD25 expression was higher in dogs with high-grade B-cell lymphoma or T-zone lymphoma than in healthy dogs [15*]. Progression-free survival was also significantly shorter in the CD25-high expressing group, indicating CD25 could be a useful prognostic marker in both species.
Ki67 is a proliferation-associated nuclear protein expressed during the active phases of the cell cycle [16]. The prognostic significance of this protein has been contradictory in humans. Poggi et al conducted a study evaluating 40 dogs with high-grade B-cell lymphoma with flow cytometry. They found that those dogs with intermediate levels had longer survival times and relapse free intervals than those with low or high levels [16]. Similar findings had been previously seen in a human study [17].
Despite the remarkable histomorphologic similarity between dogs and humans in many of the subtypes of lymphoma, Thomas et al. showed that at least in follicular lymphoma, the second most common subtype in human lymphoma which is much rarer in dogs, the molecular pathogenesis may not be the same [18*]. In humans, the constitutive expression of the anti-apoptotic bcl2 protein is induced in most cases by a chromosomal translocation. Evaluation of ten canine follicular lymphomas with IHC and genomic analysis found that while there was marked heterogeneity in the bcl2 protein expression as seen in humans, none had the hallmark translocation of human follicular lymphoma[18*]. Genomic copy number aberrations were also infrequent. The authors suggested that there may be alternative molecular mechanisms other than BCL2 translocation involved in the pathogenesis of follicular lymphoma. This illustrates how comparative oncology differences can contribute to a more comprehensive understanding of the underlying disease processes.
Improving diagnostic and minimal residual disease (MRD) detection methodologies
There have been efforts to advance the methodologies available for the detection of canine lymphoma (Figure 4). Flow cytometry was analytically and diagnostically validated for quantifying canine large B-cells in peripheral blood and bone marrow [19*]. As the degree of bone marrow infiltration has been shown to have prognostic significance in dogs with large B-cell lymphoma [20], this study validates the use of flow cytometry for this purpose. MRD is becoming an increasingly important predictive feature being incorporated into human lymphoma treatment strategies. In order to further improve the sensitivity of detecting residual populations of canine tumor cells after clinical remission, real-time quantitative PCR (RT-qPCR) was employed by designing primers and probes based on neoplastic clones from each individual canine patient [21**]. As in humans, this technique was found to be a useful prognostic indicator, a marker of treatment efficacy, and a predictor of relapse for dogs with lymphoma.
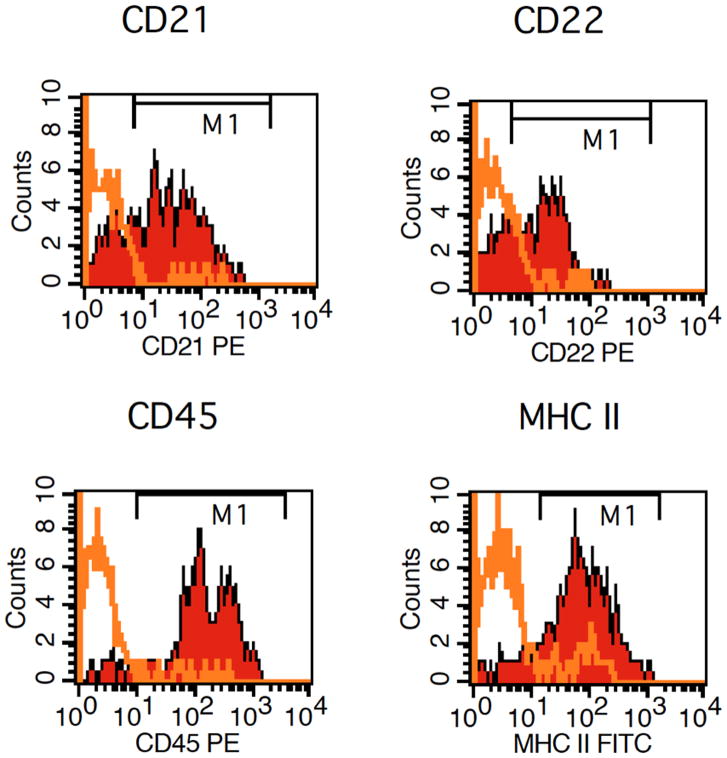
Figure 4.
Flow cytometry of a fine needle aspirate from an enlarged canine lymph node. Lymphocytes in this case expressed CD21, CD22, CD45, and MHC II indicating a B-cell lineage. Isotype-matched, normal IgG controls were appropriately negative (data not shown). Images courtesy of Dr. Tracy Stokol.
Expansion of genomic and proteomic data
In human medicine, gene expression profiling has led to the identification of two molecularly distinct forms of DLBCL with prognostic significance. Patients with germinal center B-cell (GCB) DLBCL express genes characteristic of germinal center B cells and have a significantly better overall survival than those with activated B-cell (ABC) DLBCL which express genes normally induced during activation of peripheral B cells [22]. While the human ABC/GCB signature genes do not separate canine lymphomas as cleanly, they have been used to generate a “canine-specific”ABC/GCB DLBCL signature that separates canine DLBCL into two prognostically distinct groups, mirroring human DLBCL [23]. The ABC/GCB differentiating genes, while different from those in human DLBCL, are nonetheless involved in the same pathways and processes as the human genes. For instance, in both species, increased expression of NF-KB pathway genes has been found in canine DLBCL [24] and associated with poor prognosis [23] and inactivating mutations of the negative NF-KB regulator TRAF3 [25] have been found. Whether this ABC/GCB grouping based on gene expression truly reflects two distinct cell types of origin in canine lymphoma is still unclear and the subject of ongoing research.
The lack of genomic annotations and lack of reagents for mining veterinary genomes, proteomes, and metabolomes have been signaled out as the most significant gaps in comparative oncology [3,8]. In an effort to make progress on this front, the largest canine genome-wide association study to date found a significant association for developing lymphoma near the MCC, MXD3, and FGFR4 genes in Golden Retrievers [11*]. The authors' model estimates that a much smaller number of cases and controls are needed than typically used in human studies to yield significant genetic associations, and this was borne out as the lymphoma locus achieved genome-wide significance with only 34 cases and 48 controls. This makes the dog an appealing model for studying and mapping complex phenotypes like cancer predisposition, as well as comparing the contributions of germline versus somatic mutations in cancer genomes. Previous exome sequencing studies in canine lymphoma have found recurrent somatic mutations in TRAF3 (20-35% of cases), FBXW7 (25%), POT1 (17%), and TP53 (16%) [26]. These genes have also been found to be recurrently mutated in human DLBCL (TRAF3, TP53) or other lymphoid malignancies (FBXW7, POT1) [27, 28]. Conversely, KMT2D/MLL2, the most common recurrently mutated gene in human DLBCL (25-30% of cases), has been reported in ∼5% of canine DLBCL. However, other recurrently mutated human DLBCL genes (e.g. PIM1, MYD88) have not been reported in canine DLBCL. This could be because the number of canine exomes reported is not yet large enough to detect less frequent mutations in these genes, or because different genes are targeted for mutation in the two species.
Other “-omics” studies have also provided valuable comparative information. A database of all proteomic studies carried out in the dog in health and in disease, the CanisOme, was also developed with the goal of advancing animal health as well as the use of dogs as models for human disease [29**]. There have been three reviews published in the past year detailing all of the gene expression, cytogenetic, epigenetic, and proteomic canine studies completed to date to which the reader is referred for more in-depth information [30-32*].
New canine lymphoid cell line resources
The availability of well-characterized canine lymphoid cell lines facilitates the selection of appropriate in vitro models for performing studies with clinical predictive value. Roode et al. characterized five canine leukemia/lymphoma cell lines with various methods and compared DNA copy number data from the cell lines with that from primary round cell tumors to determine if they represented the primary tumors and which subtype they most resembled [33**]. The Flint Animal Cancer Center (FACC) assembled a panel of canine cancer cell lines with associated phenotypic and genotypic information as a resource for researchers in comparative oncology [34**]. There have also been two new canine lymphoid cell lines established from a B-cell leukemia [35] and natural killer T-cell lymphoma [36*], expanding the repertoire of cell lines available for study.
Treatment: using dogs for preclinical data in human drug development
Targeted treatments such as therapeutic antibodies have become mainstream in treating human lymphoma due to their improved efficacy, and this has fostered great interest in expanding to other directed therapies to further improve patient survival. Here we discuss how, as a proof of concept, spontaneous canine lymphoma has been used as a model in preclinical trials to test novel immunotherapies and other targeted drugs.
Immunotherapy
The central rationale behind immunotherapy is that the immune system can be harnessed to recognize and eradicate neoplastic cells. There have been various methodologies employed to accomplish this including the use of T-cell checkpoint inhibitors, engineered T cells, cancer vaccines, and anti-B- and T-cell antibodies [37**]. In order to model immunotherapeutic interventions in the dog, a detailed understanding of the cellular immune response is required but still lacking. Barth et al characterized the major histocompatibility complex (MHC) binding motifs in the dog, which were similar to the human counterpart [38*]. Chimeric Antigen Receptor T cells (CARs) are engineered proteins combining the effector function of T cells with the antigen specificity of a monoclonal antibody to redirect T cell specificity [39*]. Panjwani et al developed the reagents and methods to expand and genetically modify canine T cells against the B-cell marker CD20 [39*]. Treatment of one dog with relapsed B-cell lymphoma showed transient antitumor activity indicating the potential for improving outcomes once persistent CAR expression is achieved.
Following the success of anti-CD20 therapeutic antibodies in treating diffuse large B-cell lymphoma in humans, numerous canine specific anti-CD20 monoclonal antibodies are currently in development [40]. Blocking the immune checkpoint CD47/SIRPa together with anti-CD20 immunotherapy elicited an augmented response in xenograft mouse models bearing canine lymphoma [41*]. As multiple CD47-blocking agents are now under investigation in human clinical trials, this in vivo data utilizing canine lymphoma will likely be informative for drug combination therapy in humans. More in-depth reviews on immunotherapy and monoclonal antibodies in veterinary medicine have been published this past year [37**, 42**].
Additional targets
Tyrosine kinase inhibitors have emerged as therapeutic agents able to target B-cell malignancies with increasing precision and potency. Acalabrutinib, a second-generation Bruton tyrosine kinase (BTK) inhibitor, exhibited in vitro activity and produced a clinical response in 5/20 dogs enrolled in a clinical trial [43*]. This preclinical in vivo data enabled acalabrutinib to move into human trials [44]. Additional drugs triggering autophagy modulation [45] and procaspase-3 activation [46] have also been studied in pet dogs with spontaneous lymphoma, leading to their use in phase I clinical trials in humans.
Conclusion
Despite the recent advances in establishing the spontaneous canine lymphoma model, large knowledge gaps remain requiring the collaboration between different disciplines and sectors. The field of comparative oncology still needs additional clinical trial results, pathologic, and genomic studies to better establish the areas of similarity between humans and dogs. Important human predictive/prognostic factors (e.g. ABC/GCB, double expressor lymphoma, myc translocation) should be studied in the canine model to establish the relevance in this species. The value of performing preclinical trials in pet dogs will be better established with additional well powered clinical studies of novel therapeutic agents, including correlative studies to demonstrate similar biologic effects for targeted and immune therapies. Integrating pet dogs into the drug development pipeline will ultimately enhance the predictability of treatment success and, in the long run, reduce the time and costs of therapeutic development.
Key Points.
The use of a spontaneous canine lymphoma model in drug development can improve the predictability of preclinical trials and reduce the time and costs required for drugs to come to market.
The concept of canine lymphoma serving as a useful model for the human form of the disease is becoming more mainstream with the development of necessary infrastructure and increased availability of funding opportunities.
Researchers this past year have sought to fill in the knowledge gaps still remaining and have provided proof-of-concept examples of the utility of the canine lymphoma model in the development of immunotherapies and other targeted therapeutics.
Acknowledgments
The authors would like to thank Drs. Tracy Stokol and Jimmy Tran for providing the cytology, histology, and flow cytometry images. The authors would also like to thank Ms. Kimberly Quen and her beloved dog, Stryder, as well as Dr. Vincent Baldanza and Mr. Mike Carroll for providing the canine patient photograph.
Financial Support And Sponsorship: DV is supported by the Office Of the Director, National Institutes Of Health of the National Institutions of Health under Award Number T32ODO011000. KLR is supported by a Mentored Research Scholar Grant in Applied and Clinical Research (MSRG-12-086-01-TBG) from the American Cancer Society and R01-CA185372-03 from the National Cancer Institute.
Footnotes
Conflicts Of Interest: KLR has consulted for Celgene. The remaining authors have no conflicts of interest.
References
- 1.Grimm D. From bark to bedside. Science. 2016 Aug 12;353(6300):638–640. doi: 10.1126/science.353.6300.638. [DOI] [PubMed] [Google Scholar]
- 2.Jacob JA. Researchers turn to canine clinical trials to advance cancer therapies. JAMA. 2016 Apr 19;315(15):1550–1552. doi: 10.1001/jama.2016.0082. [DOI] [PubMed] [Google Scholar]
- 3.Pang LY, Argyle DJ. Veterinary oncology: biology, big data and precision medicine. Vet J. 2016 Jul;213:38–45. doi: 10.1016/j.tvjl.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Fenger JM, Rowell JL, Zapata I, et al. Dog models of naturally occurring cancer. In: Martic-Kehl MI, Schubiger PA, editors. Animal models for human cancer: discovery and development of novel therapeutics. New Jersey: John Wiley & Sons; 2016. pp. 153–203. [Google Scholar]
- 5.Zandvliet M. Canine lymphoma: a review. Vet Q. 2016 Jun;36(2):76–104. doi: 10.1080/01652176.2016.1152633. [DOI] [PubMed] [Google Scholar]
- 6.Seelig DM, Avery AC, Ehrhart E, Linden MA. The comparative diagnostic features of canine and human lymphoma. Veterinary Sciences. 2016;3(2):1–29. doi: 10.3390/vetsci3020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner HL, Fenger JM, London CA. Dogs as a model for cancer. Annu Rev Anim Biosci. 2016;4:199–222. doi: 10.1146/annurev-animal-022114-110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc AK, Breen M, Choyke P, Dewhirst M, et al. Perspectives from man's best friend: National Academy of Medicine's Workshop on Comparative Oncology. Sci Transl Med. 2016 Feb 3;8(324):1–4. doi: 10.1126/scitranslmed.aaf0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Nass SJ, Gorby H. The role of clinical studies for pets with naturally occurring tumors in translational cancer research: workshop summary. Washington (DC): National Academy of Sciences; p. 2015. This summarizes the discussions held at the workshop by the US National Academies of Sciences, Engineering, and Medicine. [PubMed] [Google Scholar]
- 10.RFA-CA-17-001: Canine immunotherapy trials and correlative studies and RFA-CA-17-002: Coordinating center for canine immunotherapy trials.
- 11*.Hayward JJ, Castelhano MG, Oliveira KC, et al. Complex disease and phenotype mapping in the domestic dog. Nat Commun. 2016 Jan 22;7:1–11. doi: 10.1038/ncomms10460. A design for efficient mapping of complex traits and disease in dogs is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S, Xu-Monette AY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–4031. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curran K, Thamm DH. Retrospective analysis for treatment of naive canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet Comp Oncol. 2016;14(Suppl 1):147–155. doi: 10.1111/vco.12163. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara S, Muroi K, Tatara R, et al. Clinical features of de novo CD25-positive follicular lymphoma. Leuk Lymphoma. 2014 Feb;55(2):307–313. doi: 10.3109/10428194.2013.806658. [DOI] [PubMed] [Google Scholar]
- 15*.Mizutani N, Goto-Koshino Y, Tsuboi M, et al. Evaluation of CD25-positive cells in relation to the subtypes and prognoses in various lymphoid tumours in dogs. Vet Immunol Immunopathol. 2016 May;173:39–43. doi: 10.1016/j.vetimm.2016.03.018. This study illustrates how a biomarker can have prognostic significance in humans as well as in dogs. [DOI] [PubMed] [Google Scholar]
- 16.Poggi A, Miniscalco B, Morello E, et al. Prognostic significance of Ki67 evaluated by flow cytometry in dogs with high-grade B-cell lymphoma. Vet Comp Oncol. 2016 Jan;21:1–10. doi: 10.1111/vco.12184. [DOI] [PubMed] [Google Scholar]
- 17.Jerkeman M, Anderson H, Dictor M, et al. Assessment of biological prognostic factors provides clinically relevant information in patients with diffuse large B-cell lymphoma--a Nordic Lymphoma Group study. Ann Hematol. 2004 Jul;83(7):414–419. doi: 10.1007/s00277-004-0855-x. [DOI] [PubMed] [Google Scholar]
- 18*.Thomas R, Demeter Z, Kennedy KA, et al. Integrated immunohistochemical and DNA copy number profiling analysis provides insight into the molecular pathogenesis of canine follicular lymphoma. Vet Comp Oncol. 2016 May;2:1–16. doi: 10.1111/vco.12227. By studying the canine form of follicular lymphoma, new insights as to the molecular mechanisms involved in the pathogenesis of the disease in humans was gained. [DOI] [PubMed] [Google Scholar]
- 19*.Riondato F, Miniscalco B, Poggi A, et al. Analytical and diagnostic validation of a flow cytometric strategy to quantify blood and marrow infiltration in dogs with large B-cell lymphoma. Cytometry B Clin Cytom. 2016 Nov;90(6):525–530. doi: 10.1002/cyto.b.21353. This study validated the use of flow cytometry for detecting canine lymphoma cells in the blood and bone marrow. [DOI] [PubMed] [Google Scholar]
- 20.Marconato L, Martini V, Aresu L, et al. Assessment of bone marrow infiltration diagnosed by flow cytometry in canine large B cell lymphoma: prognostic significance and proposal of a cut-off value. Vet J. 2013 Sep;197(3):776–781. doi: 10.1016/j.tvjl.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 21**.Sato M, Yamazaki J, Goto-Koshino Y, et al. Minimal residual disease in canine lymphoma: an objective marker to assess tumour cell burden in remission. Vet J. 2016 Sep;215:38–42. doi: 10.1016/j.tvjl.2016.05.012. RT-qPCR was shown to be a highly sensitive tool for detecting neoplastic cells in canine patients after clinical remission. [DOI] [PubMed] [Google Scholar]
- 22.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 23.Richards KL, Motsinger-Reif AA, Chen H, et al. Gene profiling of canine B-cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res. 2013;73(16):5029–5039. doi: 10.1158/0008-5472.CAN-12-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudaliar MA, Haggart RD, Miele G, et al. Comparative gene expression profiling identifies common molecular signatures of NF-kappaB activation in canine and human diffuse large B cell lymphoma (DLBCL) PLoS One. 2013;8(9):e72591. doi: 10.1371/journal.pone.0072591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushell KR, Kim Y, Chan FC, et al. Genetic inactivation of TRAF3 in canine and human B-cell lymphoma. Blood. 2015;125(6):999–1005. doi: 10.1182/blood-2014-10-602714. [DOI] [PubMed] [Google Scholar]
- 26.Elvers I, Turner-Maier J, Swofford R, et al. Exome sequencing of lymphomas from three dog breeds reveals somatic mutation patterns reflecting genetic background. Genome Res. 2015;25(11):1634–1645. doi: 10.1101/gr.194449.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin RD, Mungall K, Pleasance E, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood. 2013;122(7):1256–1265. doi: 10.1182/blood-2013-02-483727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Grubora V, Love CL, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2013;110(4):1398–1403. doi: 10.1073/pnas.1205299110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Fernandes M, Rosa N, Esteves E, et al. CanisOme--The protein signatures of Canis lupus familiaris diseases. J Proteomics. 2016 Mar 16;136:193–201. doi: 10.1016/j.jprot.2016.01.005. This database condenses the proteomic data available for 549 canine proteins associated with 63 diseases and 33 dog breeds. [DOI] [PubMed] [Google Scholar]
- 30*.Aresu L. Canine lymphoma, more than a morphological diagnosis: what we have learned about diffuse large B-Cell lymphoma. Front Vet Sci. 2016 Aug 31;3:1–5. doi: 10.3389/fvets.2016.00077. The recent findings in the fields of gene expression profiling, cytogenetics, and epigenetics are reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Morris JS. Genomic and proteomic profiling for cancer diagnosis in dogs. Vet J. 2016 Sep;215:101–109. doi: 10.1016/j.tvjl.2016.01.003. The author provides a detailed description of the molecular profiling and proteomic studies performed on canine blood and lymphoid tissue. [DOI] [PubMed] [Google Scholar]
- 32*.Ceciliani F, Roccabianca P, Giudice C, Lecchi C. Application of post-genomic techniques in dog cancer research. Mol Biosyst. 2016 Aug 16;12(9):2665–2679. doi: 10.1039/c6mb00227g. This review focuses on the proteomic techniques performed to date on canine tissues. [DOI] [PubMed] [Google Scholar]
- 33**.Roode SC, Rotroff D, Richards KL, et al. Comprehensive genomic characterization of five canine lymphoid tumor cell lines. BMC Vet Res. 2016 Sep 17;12:1–16. doi: 10.1186/s12917-016-0836-z. The detailed characterization of canine lymphoid cell lines performed in this study will facilitate the selection of appropriate in vitro models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Fowles JS, Dailey DD, Gustafson DL, et al. The Flint Animal Cancer Center (FACC) canine tumour cell line panel: a resource for veterinary drug discovery, comparative oncology and translational medicine. Vet Comp Oncol. 2016 May;19:1–12. doi: 10.1111/vco.12192. The panel of cancer cell lines assembled by the authors contain phenotypic and genotypic information that will serve as a valuable resource for investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlak A, Ziolo E, Kutkowska J, et al. A novel canine B-cell leukaemia cell line. Establishment, characterisation and sensitivity to chemotherapeutics. Vet Comp Oncol. 2016 Aug;9:1–14. doi: 10.1111/vco.12257. [DOI] [PubMed] [Google Scholar]
- 36*.Bonnefont-Rebeix C, Fournel-Fleury C, Ponce F, et al. Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology. Immunobiology. 2016 Jan;221(1):12–22. doi: 10.1016/j.imbio.2015.08.007. The new canine cell line established in this study is the first one with natural killer cell characteristics. [DOI] [PubMed] [Google Scholar]
- 37**.Regan D, Guth A, Coy J, Dow S. Cancer immunotherapy in veterinary medicine: current options and new developments. Vet J. 2016 Jan;207:20–28. doi: 10.1016/j.tvjl.2015.10.008. This article provides a thorough review of the mechanisms of action of immunotherapies and the latest updates in this field in veterinary species. [DOI] [PubMed] [Google Scholar]
- 38*.Barth SM, Schreitmuller CM, Proehl F, et al. Characterization of the canine MHC Class I DLA-88*50101 peptide binding motif as a prerequisite for canine T cell immunotherapy. PLoS One. 2016 Nov 28;11(11):1–19. doi: 10.1371/journal.pone.0167017. This study contributed immunology knowledge lacking in dogs that is necessary for the successful development of immunotherapies in this species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Panjwani MK, Smith JB, Schutsky K, et al. Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor T cells in dogs with spontaneous B cell lymphoma. Mol Ther. 2016 Sep;24(9):1602–1614. doi: 10.1038/mt.2016.146. The authors were able to develop the reagents and methods needed to create CARs from canine T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JS, Withers SS, Modiano JF, et al. Canine cancer immunotherapy studies: linking mouse and human. J Immunother Cancer. 2016 Dec 20;4:1–11. doi: 10.1186/s40425-016-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Weiskopf K, Anderson KL, Ito D, et al. Eradication of canine diffuse large B-cell lymphoma in a murine xenograft model with CD47 blockade and anti-CD20. Cancer Immunol Res. 2016 Dec;4(12):1072–1087. doi: 10.1158/2326-6066.CIR-16-0105. This study is informative as to how different immunotherapies can be combined for a synergistic effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Beirao BC, Raposo T, Jain S, et al. Challenges and opportunities for monoclonal antibody therapy in veterinary oncology. Vet J. 2016 Dec;218:40–50. doi: 10.1016/j.tvjl.2016.11.005. This is an in-depth review on the development of monoclonal antibodies in veterinary medicine. [DOI] [PubMed] [Google Scholar]
- 43*.Harrington BK, Gardner HL, Izumi R, et al. Preclinical evaluation of the novel BTK inhibitor acalabrutinib in canine models of B-cell non-Hodgkin lymphoma. PLoS One. 2016 Jul 19;11(7):1–18. doi: 10.1371/journal.pone.0159607. The preclinical data obtained in canine lymphoma patients in this study enabled acalabrutinib to move into human phase I clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016 Mar 9;9:1–4. doi: 10.1186/s13045-016-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2016 Jan 7;35(1):1–11. doi: 10.1038/onc.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Botham RC, Roth HS, Book AP, et al. Small-molecule procaspase-3 activation sensitizes cancer to treatment with diverse chemotherapeutics. ACS Cent Sci. 2016 Aug 24;2(8):545–559. doi: 10.1021/acscentsci.6b00165. [DOI] [PMC free article] [PubMed] [Google Scholar]