Abstract
Resistant starch (RS) is a dietary fermentable fiber that decreases body fat accumulation, and stimulates the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) in rodents. GLP-1 and PYY are gut secreted hormones with anti-obesity effect. Thus, blocking the signals of increased GLP-1 and PYY may also block the effect of dietary RS on body fat. In a ten-week study, C57BL/6J and GLP-1 receptor null (GLP-1R KO) mice were fed control or 30% RS diet, and received daily intraperitoneal injection of either saline or PYY receptor antagonist (BIIE 0246, 20ug/kg body weight). Dietary RS significantly decreased body fat accumulation only in wild type mice that has saline injection, but not in GLP-1R KO mice. PYY receptor antagonist diminished RS action on body fat in wild type mice, but did not interfere with GLP-1R KO mice response to RS. Regardless of genotype and injection received, all RS fed mice had increased cumulative food intake, cecal fermentation, and mRNA expression of proglucagon and PYY. Thus, our results suggest that increased GLP-1 and PYY is important in RS effects on body fat accumulation.
Keywords: Antagonist, Dietary Fiber, GLP-1 receptor, Obesity, PYY receptor
Resistant starch (RS) is a fermentable dietary fiber used as a carbohydrate source in food. Among many beneficial effects of dietary RS [1-9], we consistently found RS fed rodents have decreased body fat and improved glucose tolerance, associated with elevated circulating GLP-1 and PYY [10-18]. GLP-1 and PYY are gut-secreted hormones with anti-obesity and anti-diabetic actions. The expressions of these two hormones are stimulated by RS through its colonic fermentation [18, 19]. When the mice failed to ferment RS diet, they also failed to show body fat loss [17]. Thus, we hypothesize that dietary RS decreases body fat accumulation through stimulating endogenous GLP-1 and PYY production. In current study, we used GLP-1 receptor null (GLP-1R KO) mice and PYY receptor antagonist to block the signaling of elevated GLP-1/PYY in RS fed mice, and measured their body fat accumulation.
Both GLP-1R KO (provided by Dr. Drucker, Department of Medicine, University of Toronto, Canada) and wild type mice (Jackson Laboratories, ME USA) were bred in the same room with free access to rodent chow and tap water. All GLP-1R KO mice were genotyped to ensure their correct genotype. Only male mice were used for study.
At the age of 8-12weeks, mice were switched to wire-bottom cages and custom made control diet for adaptation of experimental conditions. The control diet was semi-purified powder diets of AIN-93G for laboratory rodents. After two weeks adaptation, based on their body weight and body fat (measured by NMR), both wild type and GLP-1RKO mice were divided into two dietary groups: control diet or 30% RS diet. The RS diet has the same micro- and macro- nutrients, and metabolizable energy density as the control diet. However, the regular corn starch (Amioca ® cornstarch, 100% amylopectin) or Hi-maize 260®, (Ingredion, Incorporated, Bridgewater, NJ) were used for control diet or RS diet as described previously[18]. The mice in each dietary group were further subdivided into receiving daily intraperitoneal injection of either saline or PYY receptor antagonist (BIIE0246, 20ug/kg body weight, Tocris Bioscience, Bristol UK). Animal protocols were approved by the Pennington Biomedical Research Center Animal Care and Use Committees (protocol # 419). There was a total of 8 groups of mice (2 types of mice × 2 diets × 2 injections) used in the study, and each group had 8-12 mice. There are 6 mice that did not gain or lost body weight because of complications associated with daily ip injection during the entire study period, and they are excluded for data analysis.
At the end of study, fat pads (epididymal fat, perirenal fat, and retroperitoneal fat) from the abdominal cavity were dissected and combined as total abdominal fat. Body fat was expressed as percent of total abdominal fat divided by disemboweled body weight (DBW). DBW was used here because of the significantly greater gastrointestinal (GI) weight in RS fed mice, and obtained by subtracting the weight of full GI tract (base3 of esophagus to anus) from the whole body weight at euthanasia.
Of note, the body fat and body weight were significantly greater in GLP-1R KO mice than wild type mice. As the aim of the current study was to investigate effect of RS diet, not to compare the physiological differences between the two types of mice, all comparisons were made within the same genotype mice. A two-way ANOVA with diet and injection as two categorical independent variables was used to determine effects of RS diet and antagonist for PYY receptor. Additionally, the RS effect was also determined by student T-test within the same genotype and same injection groups.
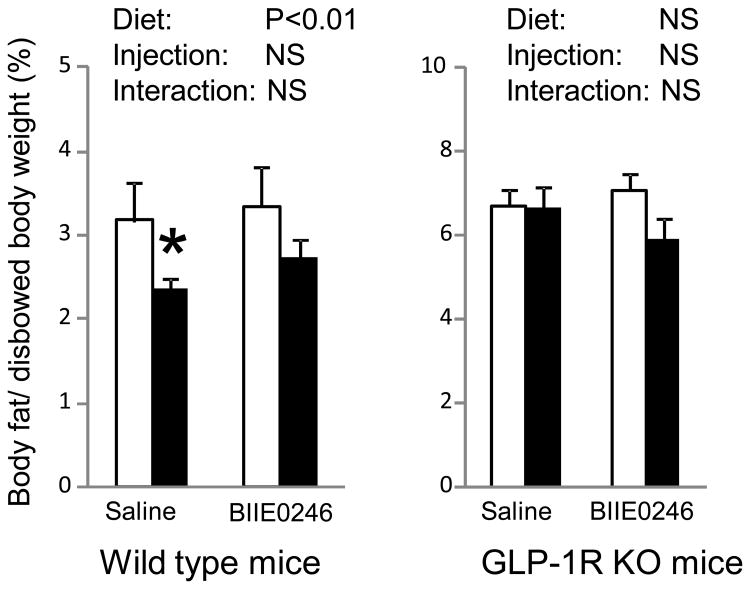
Dietary RS significantly reduced body fat only in wild type mice that had saline injection but not in GLP-1R KO mice, although GLP-1R KO mice had higher % of body fat (Figure 1). BIIE0246 injection had no effect on body fat in both wild type mice and GLP-1R KO mice. When data were analyzed by student T-test for wild type mice that received the same type of injection, dietary RS reduced body fat in mice that had saline injection (P<0.05); and the reduction is not statistically significant for mice that had BIEE0246 injection.
Figure 1.
Body fat was reduced in wild type mice, but not in GLP-1R KO mice with RS feeding. PYY receptor antagonist (BIIE0246) injection did not change the effects of dietary RS on body fat. Mice were fed control diet (□) or 30% RS diet (■) and had a daily injection of saline or BIIE0246 for 10 weeks. Body fat was measured by excision of fat depots at euthanasia. Data are mean ± SE (n=8-12 per group). “NS” indicates no statistical significance analyzed by two-way ANOVA. * indicates the RS-fed group had significant lower body fat than its relative control group.
We also measured food intake three times per week during the study period. Food intake was determined by measuring food jar weight and spillage for each mouse and calculated as cumulative food intake. Over the ten week study period, dietary RS significantly increased cumulative food intake when compared with their respective controls (Table 1). At the euthanasia, body weight, DBW, and liver weight were not different between RS-fed mice and controls. The fermentation indicators (weights of full GI tract, full and empty cecum) are all significantly increased in RS-fed mice (Table 1). Also, GLP-1RKO and BIIE0246 injection did not interfere with dietary RS fermentation in the gut.
Table 1.
Food intake, Body weight, Disembowled body weight (DBW), Liver weight, and indicators of fermentation in wild type and GLP-1R KO mice fed control or RS diet at the end of ten week study. Data are mean ± SE with group of 8-12 mice.
| Food intake (grams) | Body weight (grams) | DBW (grams) | Liver weight (grams) | Full GI weight (mg) | Full cecal weight (mg) | Empty cecal weight (mg) | |||
|---|---|---|---|---|---|---|---|---|---|
| Wild | Saline | C | 253.2±8.8 | 27.4±1.2 | 25.5±0.9 | 1.18±0.05 | 1725±104 | 238±25 | 67±5 |
| RS | 270.4±3.4* | 26.9±0.5 | 24.9±2.0 | 1.16±0.05 | 2332±95* | 592±55* | 116±11* | ||
| Antagonist | C | 254.7±5.3 | 27.2±1.0 | 25.5±0.9 | 1.15±0.08 | 1575±64 | 178±16 | 61±6 | |
| RS | 277.4±6.3* | 26.8±0.4 | 24.4±0.4 | 1.18±0.04 | 2343±120* | 586±74* | 113±11* | ||
| GLP-1RKO | Saline | C | 256.9±4.7 | 32.6±1.0 | 28.4±2.7 | 1.26±0.12 | 1744±166 | 266±31 | 83±10 |
| RS | 274.2±6.4* | 33.1±1.0 | 30.1±0.9 | 1.32±0.04 | 3135±139* | 1179±91* | 211±19* | ||
| Antagonist | C | 254.1±5.9 | 33.0±1.6 | 31.0±1.6 | 1.33±0.08 | 1937±96 | 298±025 | 95±7 | |
| RS | 278.1±5.2* | 32.9±1.3 | 30.2±1.3 | 1.34±0.04 | 2801±128* | 878±110* | 169±12* |
P<0.05 compared with relative control in the same genotype of mice that received the same type of injection. There was no interaction between diet and type of injection.
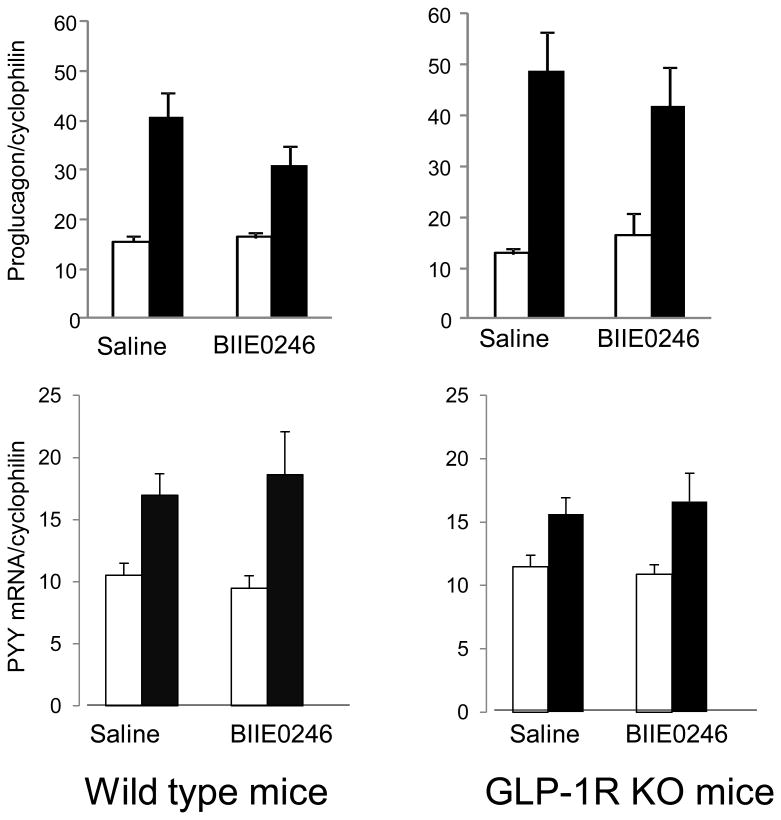
To confirm that all RS fed mice have increased GLP-1 and PYY, cecal epithelial cells were collected at euthanasia by gently scraping the inner surface of cecum for total RNA extraction and measuring mRNA of proglucagon (the gene encodes GLP-1) and PYY as previously described [19]. Both proglucagon and PYY mRNA expression were significantly increased in all RS fed mice (P<0.01, Fig.2). Serum active GLP-1 and total PYY were also measured by Milliplex mouse gut hormone magnetic bead panel (Millipore Corporation). The results show that most control mice had under the detection limit level of GLP-1 and PYY, while most RS fed mice had above detection levels of GLP-1 and PYY. Due to limited serum volume, the measurements could not be repeated. Thus, the data were analyzed by a nonparametric method. All RS fed mice had increased serum levels of active GLP-1 and total PYY (data not shown), consistent with our previous observation that when rodents can ferment RS diet, their endogenous GLP-1 and PYY production will be increased [11, 13, 18].
Figure 2.
Dietary resistant starch increases cecal proglucagon and PYY mRNA expressions measured by real-time PCR. Data are Mean±SE (n=8-11 per group). For both type of mice, the effect of diet was P<0.01, the effect of injection and interaction were not statistically significant.
The aim of this study was to test the importance of increased GLP-1 and PYY in effect of dietary RS on body fat. Thus, we intended to block actions of increased GLP-1 and PYY in RS fed mice and examine if the RS diet was still effective. Injection of PYY receptor antagonist BIIE0246 only blocks action of PYY temporarily as half-life of BIIE0246 is less than 3 hours [20]. Although the dosage of BIIE 0246 used in the current study blocked the effect of PYY on food intake for several hours [21], it is unlikely to fully block the action of a day-long increase of PYY [18] in RS-fed mice. Therefore, injection of PYY receptor antagonist did not completely abolish the dietary RS effect of reducing body fat. In contrast, when the GLP-1 signal is blocked completely in GLP-1R KO mice, dietary RS does not decrease body fat accumulation.
It is well known that GLP-1 and PYY decrease food intake at pharmacological dosages, which are ranged at level of ng/ml or nmole or even higher [22-24]. Interestingly, all RS-fed mice had increased cumulative food intake in the current study despite their elevated GLP-1 and PYY. We also reported the same observation previously [17]. Of note, GLP-1 and PYY do not affect food intake when used at the dosage below the effective pharmacological dosages [22, 24]. The increased GLP-1 and PYY are in pg/ml or pmole range in RS fed rodents [11, 13-16, 18], which is much lower than the pharmacological dosages used to decrease food intake. Additionally, although having consistently elevated the lower levels of GLP-1 and PYY, RS fed rodents did not have peaks of these hormones after meals [18], which are believed to affect satiety. Our results also suggest that 30% RS in the diet did not cause adverse effects that usually lead to appetite suppression and body weight reduction.
The mechanism of increased food intake in RS fed mice still needs further investigation. Considering the cumulative food intake was recorded over the ten week period, the increases were small and did not hamper lower accretion of body fat in RS fed wild type mice. We have previously demonstrated that respiratory quotient (RQ) was significantly decreased in RS fed mice, an indicator that these mice utilize more fat than carbohydrate for metabolic energy supply[17]. This hypothesized downstream signaling pathway of GLP-1 and PYY also needs further investigation.
In summary, our results indicate GLP-1 and PYY play an important role in the dietary RS effect on body fat reduction.
Acknowledgments
This work was supported by NIH R21 DK073403.
Glossary
- RS
Resistant starch
- GLP-1
Glucagon-like peptide-1
- PYY
Peptide YY
- DBW
Disemboweled body weight
References
- 1.Higgins JA, Brown IL. Resistant starch: a promising dietary agent for the prevention/treatment of inflammatory bowel disease and bowel cancer. Curr Opin Gastroenterol. 2013;29:190–194. doi: 10.1097/MOG.0b013e32835b9aa3. [DOI] [PubMed] [Google Scholar]
- 2.Higgins JA. Resistant starch and energy balance: impact on weight loss and maintenance. Crit Rev Food Sci Nutr. 2014;54:1158–1166. doi: 10.1080/10408398.2011.629352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hullar MA, Burnett-Hartman AN, Lampe JW. Gut microbes, diet, and cancer. Cancer Treat Res. 2014;159:377–399. doi: 10.1007/978-3-642-38007-5_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asp NG. Resistant starch--an update on its physiological effects. Adv Exp Med Biol. 1997;427:201–210. doi: 10.1007/978-1-4615-5967-2_21. [DOI] [PubMed] [Google Scholar]
- 5.Bird AR, Conlon MA, Christophersen CT, Topping DL. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes. 2011;1:423–431. doi: 10.3920/BM2010.0041. [DOI] [PubMed] [Google Scholar]
- 6.Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res. 2005;49:560–570. doi: 10.1002/mnfr.200500025. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DJ, Kendall CW. Resistant starches. Curr Opin Gastroenterol. 2000;16:178–183. doi: 10.1097/00001574-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Garcia TA, McCutcheon KL, Francis AR, Keenan MJ, et al. The effects of resistant starch on gastrointestinal organs and fecal output in rats. FASEB Journal. 2003;17 [Google Scholar]
- 9.Al-Tamimi EK, Seib PA, Snyder BS, Haub MD. Consumption of Cross-Linked Resistant Starch (RS4(XL)) on Glucose and Insulin Responses in Humans. J Nutr Metab. 2010;2010 doi: 10.1155/2010/651063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan MJ, Janes M, Robert J, Martin RJ, et al. Resistant starch from high amylose maize (HAM-RS2) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity (Silver Spring) 2013;21:981–984. doi: 10.1002/oby.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, et al. Effects of Resistant Starch, A Non-digestible Fermentable Fiber, on Reducing Body Fat. Obesity (Silver Spring) 2006;14:1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 12.Vidrine K, Ye J, Martin RJ, McCutcheon KL, et al. Resistant starch from high amylose maize (HAM-RS2) and Dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20501. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Keenan MJ, Fernandez-Kim SO, Pistell PJ, et al. Dietary resistant starch improves selected brain and behavioral functions in adult and aged rodents. Mol Nutr Food Res. 2013;57:2071–2074. doi: 10.1002/mnfr.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, et al. High-Amylose Resistant Starch Increases Hormones and Improves Structure and Function of the Gastrointestinal Tract: A Microarray Study. J Nutrigenet Nutrigenomics. 2012;5:26–44. doi: 10.1159/000335319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen L, Keenan MJ, Martin RJ, Tulley RT, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17:40–45. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakizaki rat. Mol Nutr Food Res. 2011;55:1499–1508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Martin RJ, Tulley RT, Raggio AM, et al. Failure to Ferment Dietary Resistant Starch in Specific Mouse Models of Obesity Results in No Body Fat Loss. J Agric Food Chem. 2009 doi: 10.1021/jf901548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Martin RJ, Tulley RT, Raggio AM, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, et al. Peptide YY and Proglucagon mRNA Expression Patterns and Regulation in the Gut. Obesity (Silver Spring) 2006;14:683–689. doi: 10.1038/oby.2006.77. [DOI] [PubMed] [Google Scholar]
- 20.Brothers SP, Saldanha SA, Spicer TP, Cameron M, et al. Selective and brain penetrant neuropeptide y y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol Pharmacol. 2010;77:46–57. doi: 10.1124/mol.109.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott CR, Small CJ, Kennedy AR, Neary NM, et al. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3-36) on food intake. Brain Res. 2005;1043:139–144. doi: 10.1016/j.brainres.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 22.Neary NM, Small CJ, Druce MR, Park AJ, et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005 doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 23.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 24.Parker JA, McCullough KA, Field BC, Minnion JS, et al. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes (Lond) 2013;37:1391–1398. doi: 10.1038/ijo.2012.227. [DOI] [PubMed] [Google Scholar]