Abstract
Background
Although a well-established causative relationship exists between smoking and several epithelial cancers, the association of smoking with metastatic progression in melanoma is not well studied. We hypothesized that smokers would be at increased risk for melanoma metastasis as assessed by sentinel lymph node (SLN) biopsy.
Methods
Data from the first international Multicenter Selective Lymphadenectomy Trial (MSLT-I) and the screening-phase of the second trial (MSLT-II) were analyzed to determine the association of smoking with clinicopathologic variables and SLN metastasis.
Results
Current smoking was strongly associated with SLN metastasis (p = 0.004), even after adjusting for other predictors of metastasis. Among 4231 patients (1025 in MSLT-I and 3206 in MSLT-II), current or former smoking was also independently associated with ulceration (p < 0.001 and p < 0.001, respectively). Compared with current smoking, never smoking was independently associated with decreased Breslow thickness in multivariate analysis (p = 0.002) and with a 0.25 mm predicted decrease in thickness.
Conclusion
The direct correlation between current smoking and SLN metastasis of primary cutaneous melanoma was independent of its correlation with tumor thickness and ulceration. Smoking cessation should be strongly encouraged among patients with or at risk for melanoma.
Smoking is a known preventable cause of cancer mortality.1 Based on estimates from the World Health Organization, by 2020, 8.4 million people will die each year from tobacco-related diseases and over 100 million people died from tobacco-related diseases in the 20th century.1, 2 Counterintuitively, smoking does not appear to be associated with an increased incidence of melanoma, and there may even be a protective effect, although these data are controversial.3–8 Few studies have evaluated the impact of smoking on the progression of primary cutaneous melanoma, and many clinical trials in oncology do not collect smoking data unless smoking is thought to contribute to disease pathogenesis.9 Studies assessing the association of smoking with nodal metastasis in melanoma are limited and predate modern methods of nodal assessment with sentinel lymph node biopsy (SNB). We undertook this study to determine if smoking is associated with the early, subclinical phase of metastasis, as assessed by a melanoma-positive sentinel lymph node (SLN).
METHODS
The Multicenter Selective Lymphadenectomy Trials are prospective international trials evaluating methods of lymph node management in patients with clinically localized cutaneous melanomas. Data for this analysis were derived from patients in the entire MSLT-I trial (NCT00275496) and the screening phase of the MSLT-II trial (NCT00297895).11 The MSLT-I study compared treatment by wide local excision alone versus wide local excision with sentinel node biopsy and immediate completion lymph node dissection for SLN metastases.11 Since only one arm of the study underwent SLN assessment, this analysis includes only that group. The screening phase of MSLT-II enrolled patients prior to SLN biopsy with clinically localized melanoma at least 1.2 mm in thickness or with invasion to at least Clark’s level IV. Patients who were found to have SLN metastases by standard pathology or multimarker quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) were eligible for entry into the randomized phase of the trial, which is currently closed to accrual and ongoing.
Smoking status was recorded at trial enrollment either via patient questionnaire or intake interview. Patients were categorized as current, former, or never smokers; the smoking group included tobacco inhaled via cigarette, pipe, cigar or other source. Subjects with incomplete data regarding smoking history, Breslow thickness, ulceration status, primary site, or receipt of SNB for MSLT-I patients were excluded (n = 2732). Subjects provided written informed consent for participation in the clinical trials and, for the current analysis, were de-identified and independently determined to be exempt from Institutional Review Board review.
Demographic and clinicopathologic factors examined in this study included age, sex, ulceration, Breslow thickness, primary site, and SLN status. These factors were compared between the three smoking groups using the Chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables such as age and Breslow thickness. Multivariate analysis was conducted for all factors associated with ulceration and again for factors associated with Breslow thickness. Finally, a logistic regression model for clinicopathologic factors associated with SLN metastasis was created (Table 3). We utilized SAS software version 9.3 (SAS Institute, Cary, NC, USA) for all analyses. A two-sided p-value of ≤ 0.05 was considered significant.
TABLE 3.
Logistic regression for SLN metastasis
| Variable | Univariable
|
Multivariable
|
||||
|---|---|---|---|---|---|---|
| p-value | OR | 95% CI | p-value | OR | 95% CI | |
| Smoking: current versus never | <0.001 | 1.63 | (1.35–1.98) | 0.004 | 1.34 | (1.10–1.65) |
| Smoking: former versus never | 0.620 | 1.05 | (0.87–1.26) | 0.474 | 1.07 | (0.89–1.30) |
| Age (continuous) | <0.001 | 0.98 | (0.98–0.99) | < 0.001 | 0.98 | (0.98–0.99) |
| Male versus female | 0.083 | 1.14 | (0.98–1.33) | 0.578 | 1.05 | (0.89–1.24) |
| Ulceration: present versus absent | <0.001 | 2.27 | (1.94–2.66) | < 0.001 | 1.83 | (1.54–2.16) |
| Breslow (continuous) | <0.001 | 1.25 | (1.20–1.30) | < 0.001 | 1.20 | (1.15–1.25) |
| Extremity versus head/neck | 0.529 | 0.93 | (0.74–1.17) | 0.584 | 1.94 | (0.73–1.19) |
| Trunk versus head/neck | 0.001 | 1.47 | (1.18–1.84) | 0.010 | 1.36 | (1.08–1.71) |
SLN sentinel lymph node, OR odds ratio, CI confidence interval
RESULTS
The 4231 subjects included 1025 patients from MSLT-I and 3206 patients from MSLT-II (Table 1). Current smokers (N = 719) were significantly younger than former smokers (N = 1100) or never smokers (N = 2412). Current smokers also had more ulcerated lesions than former smokers or never smokers. In addition, former smokers were more likely to be male than never smokers or current smokers, and never smokers had thinner lesions than former smokers or current smokers. There was a difference in primary site distribution between head/neck, extremity and truncal lesions based on smoking status; however, there was no difference in melanoma site between current and former smokers or between former and never smokers. Finally, current smokers were significantly more likely to have SLN metastasis when all three groups were compared (Fig. 1), or when current smokers were compared separately to never smokers, and even former smokers.
TABLE 1.
Logistic regression model for ulceration
| Variable | p-value | OR | 95% CI |
|---|---|---|---|
| Smoking: former versus current | <0.001 | 0.68 | 0.55, 0.85 |
| Smoking: never versus current | <0.001 | 0.70 | 0.58, 0.84 |
| Age (continuous) | 0.001 | 1.01 | 1.00, 1.02 |
| Male versus female | 0.618 | 1.04 | 0.89, 1.21 |
| Breslow (continuous) | <0.001 | 1.44 | 1.38, 1.50 |
| Extremity versus head/neck | 0.356 | 1.11 | 0.89, 1.37 |
| Trunk versus head/neck | 0.005 | 1.35 | 1.09, 1.66 |
OR odds ratio, CI confidence interval
FIG. 1.
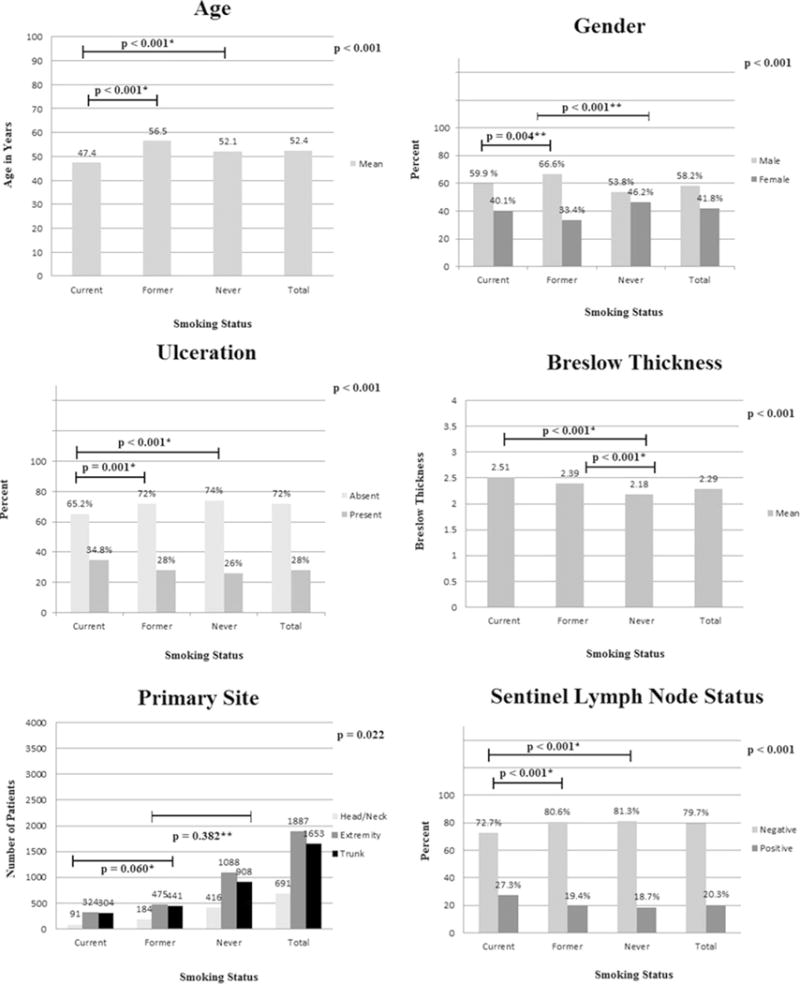
Demographic and histopathologic characteristics. Analysis of clinicopathologic factors associated with sentinel node metastasis in patients from MSLT-I and MSLT-II. p-Values in the upper right hand corner pertain to the group overall. *Denotes comparison of the selected group versus current smokers, ** denotes comparison of the selected group versus former smokers, *** denotes comparison of the selected group versus never smokers
Logistic regression analysis showed that both current smoking and former smoking were independently associated with ulceration (Table 1). Multivariate regression analysis revealed an association between smoking and Breslow thickness (Table 2); never smoking was associated with a 0.25 mm decrease in Breslow thickness relative to current smoking, independent of other variables. Breslow thickness was 1.2 mm higher if the lesion was ulcerated, and thickness increased by 0.01 mm with each year of age. Breslow thickness was 0.15 mm less for females than males.
TABLE 2.
Regression model for Breslow thickness
| Variable | p-value | Parameter estimate | 95% CI |
|---|---|---|---|
| Smoking: never versus current | 0.002 | −0.25 | −0.14, −0.09 |
| Smoking: former versus current | 0.219 | −0.11 | −0.29, 0.07 |
| Ulceration | <0.001 | 1.20 | 1.08, 1.33 |
| Trunk primary versus head/neck | 0.880 | 0.01 | −0.15, 0.18 |
| Extremity versus head/neck | 0.098 | −0.14 | −0.31, 0.03 |
| Age (continuous) | 0.004 | 0.010 | 0.00, 0.01 |
| Female versus male | 0.015 | −0.15 | −0.27, −0.03 |
CI confidence interval
On univariable analysis, current smoking (compared with never smoking) was significantly associated with SLN positivity, and this factor remained significant at multivariable analysis (Table 3). In these analyses, increasing age was significantly associated with a small decrement in risk of SLN positivity. Ulceration and Breslow thickness were also associated with increased SLN positivity in multivariable analysis. In this analysis, there was also a significant shift towards SLN positivity in truncal tumors as opposed to head and neck tumors.
DISCUSSION
In a large sample of patients from two international, prospective, randomized clinical trials, current smoking was strongly associated with SLN metastasis, even after adjusting for other predictors of melanoma metastasis. Furthermore, smoking was also associated with other adverse prognostic features, including increasing Breslow depth and ulceration. To our knowledge, this is the largest study examining smoking as a factor predicting metastasis in cutaneous melanoma, and the only study to examine this phenomenon with regard to SLN metastasis. These results suggest smoking may promote early dissemination of melanoma to regional sites.
Although in the past there has been some suggestion that smoking was associated with an increased propensity to early metastasis in some malignancies, the literature is somewhat limited. In many large databases and clinical trials, smoking status is not assessed or recorded at initial melanoma diagnosis, and it is also generally undermonitored during follow-up.9, 12, 13 Despite extensive literature examining clinical and pathologic factors that are related to SLN metastasis in melanoma, to our knowledge no study has examined the impact of smoking on this process; however, some studies have examined the relationship of smoking to nodal metastases in other malignancies, including bladder cancer, renal cell cancer, and head and neck cancers.14–18 Lynch and colleagues identified a greater than 10 pack-year smoking history as a risk factor for contralateral nodal recurrence in tonsillar cancer.15 In a predictive model assessing clinicopathologic, molecular, and genetic characteristics in clear cell renal cell carcinoma, Kroeger and colleagues identified smoking as independently associated with increased lymphatic spread.18 Lung, pancreatic, breast, prostate, colon, bladder, esophageal, and oral cancers have also been reported to have an association with smoking and the potential for either metastatic spread or progression.17–25
The mechanisms responsible for this effect are not yet known, but several plausible hypotheses exist. Smoking may increase SLN metastasis by enhancing immunosuppressive factors in the tumor’s microenvironment.26–31 For example, carboxyhemoglobinemia from carbon monoxide contained in tobacco smoke produces a prolonged hypoxic state, and tumor hypoxia is associated with increased metastasis and mortality in several malignancies.2 Hypoxia can also upregulate programmed death-ligand 1 (PD-L1) expression on myeloid suppressor cells, macrophages, dendritic cells and tumor cells, promote regulatory T-cell abundance, and increase lactate secretion.27–30
Our analysis is limited by its reliance on self-reported smoking history and the lack of prospective, objective, and systematized quantitative smoking assessments. Such information, obtained from very large patient cohorts, might be able to demonstrate a dose-response relationship, and would be an interesting area of future research.32, 33 Future study and clinical databases should include more detailed quantitative smoking assessments such as ‘pack year’ data, number of smoking years, and time interval since quitting. In addition, more precise details on type of tobacco products used should be recorded in the future. A dose response relationship with regard to SLN metastasis was not identified in this study; however, quantitative smoking history was incomplete and deemed less reliable. In the data set, 11.6% of patients were missing pack-year data, 7.3% were missing data regarding cigarettes consumed per day, and 10% were missing data pertaining to years smoked. We also did not capture data on ‘passive smoking’ (exposure to cigarette smoke produced by others). Utilization of biomarkers for assessment of smoke exposure might help overcome these limitations. There may also be other as yet unquantified variables, such as ultraviolet radiation exposure, that could be associated with smoking behavior which were not captured in our data set.34 Unassessed social factors potentially associated with smoking behavior could also be responsible for our findings. These may include decreased health literacy, poor treatment compliance, and diminished access to medical care, among others. Such alternate explanations must be considered given the noted adverse prognostic features identified in smokers without concomitant increase in melanoma incidence. While it is generally assumed these social factors affect trial patients less frequently than the general population, we cannot discount alternate etiologies of increased tumor thickness, ulceration, and nodal metastasis.
CONCLUSIONS
Despite these weaknesses, our findings indicate that current smoking is associated with an increase in the risk of SLN metastasis in melanoma patients and that this effect is independent of other risk factors. We therefore believe that smoking at the time of melanoma diagnosis should be considered an important risk stratification factor.35 Smoking should be investigated as a factor contributing to early metastasis of melanoma and possibly other tumor types, and should be considered for inclusion in databases in the future. The limited impact of former smoking on SLN positivity suggests a transient and likely reversible effect of smoking on the metastatic potential of melanoma. There are obviously many reasons why current smokers should quit, but this analysis provides an additional rationale for doing so for those who are at risk for melanoma development, particularly those recently diagnosed with the disease.
Acknowledgments
This study was supported by Grants CA189163 and CA29605 from the National Cancer Institute, and by funding from the Amyx Foundation, Inc. Boise, ID, USA; the Borstein Family Foundation, Los Angeles, CA, USA; Dr Miriam and Sheldon G. Adelson Medical Research Foundation, Boston, MA, USA; and the John Wayne Cancer Institute Auxiliary, Santa Monica, CA, USA. Dr Maris S. Jones is the Harold McAlister Charitable Foundation Fellow. The content of this report is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Footnotes
Presented in part at the Society of Surgical Oncology Annual Meeting, held in Boston, MA, USA, 2–5 March 2016
References
- 1.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370(1):60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 2.Twombly R. World Health Organization takes on ‘tobacco epidemic’. J Natl Cancer Inst. 2002;94(9):644–46. doi: 10.1093/jnci/94.9.644. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO, Hannan LM, Gapstur SM, Thun MJ. Cigarette smoking and the risk of incident and fatal melanoma in a large prospective cohort study. Cancer Causes Control. 2011;22(6):937–42. doi: 10.1007/s10552-011-9766-z. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DM, Sigurdson A, Doody MM, Rao RS, Linet MS. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control. 2003;14(9):847–57. doi: 10.1023/b:caco.0000003839.56954.73. [DOI] [PubMed] [Google Scholar]
- 5.Song F, Qureshi AA, Gao X, Li T, Han J. Smoking and risk of skin cancer: a prospective analysis and a meta-analysis. Int J Epidemiol. 2012;41(6):1694–705. doi: 10.1093/ije/dys146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odenbro A, Gillgren P, Bellocco R, Boffetta P, Hakansson N, Adami J. The risk for cutaneous malignant melanoma, melanoma in situ and intraocular malignant melanoma in relation to tobacco use and body mass index. Br J Dermatol. 2007;156(1):99–105. doi: 10.1111/j.1365-2133.2006.07537.x. [DOI] [PubMed] [Google Scholar]
- 7.Henderson MT, Kubo JT, Desai M, et al. Smoking behavior and association of melanoma and nonmelanoma skin cancer in the Women’s Health Initiative. J Am Acad Dermatol. 2015;72(1):190–91. doi: 10.1016/j.jaad.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson CA, Zhang ZF, Arah OA. Competing risk bias to explain the inverse relationship between smoking and malignant melanoma. Eur J Epidemiol. 2013;28(7):557–67. doi: 10.1007/s10654-013-9812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2287–93. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 10.Shaw HM, Milton GW. Smoking and the development of metastases from malignant melanoma. Int J Cancer. 1981;28(2):153–56. doi: 10.1002/ijc.2910280207. [DOI] [PubMed] [Google Scholar]
- 11.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitas F, Weber MF, Egger S, Yap S, Chiew M, O’Connell D. Smoking cessation after cancer. J Clin Oncol. 2014;32(32):3593–95. doi: 10.1200/JCO.2014.55.9666. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein AO, Ripley-Moffitt CE, Pathman DE, Patsakham KM. Tobacco use treatment at the U.S. National Cancer Institute’s designated Cancer Centers. Nicotine TobRes. 2013;15(1):52–58. doi: 10.1093/ntr/nts083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom PJ, Alkhateeb S, Trottier G, et al. Sex differences in bladder cancer outcomes among smokers with advanced bladder cancer. BJU Int. 2012;109(1):70–76. doi: 10.1111/j.1464-410X.2011.10371.x. [DOI] [PubMed] [Google Scholar]
- 15.Lynch J, Lal P, Schick U, et al. Multiple cervical lymph node involvement and extra-capsular extension predict for contralateral nodal recurrence after ipsilateral radiotherapy for squamous cell carcinoma of the tonsil. Oral Oncol. 2014;50(9):901–06. doi: 10.1016/j.oraloncology.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Yoneyama R, Aoshiba K, Furukawa K, et al. Nicotine enhances hepatocyte growth factor-mediated lung cancer cell migration by activating the alpha7 nicotine acetylcholine receptor and phosphoinositide kinase-3-dependent pathway. Oncology Lett. 2016;11(1):673–77. doi: 10.3892/ol.2015.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren GW, Singh AK. Nicotine and lung cancer. J Carcinog. 2013;12:1. doi: 10.4103/1477-3163.106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroeger N, Seligson DB, Klatte T, et al. Clinical, molecular, and genetic correlates of lymphatic spread in clear cell renal cell carcinoma. Eur Urol. 2012;61(5):888–95. doi: 10.1016/j.eururo.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180(1):31–37. doi: 10.1016/j.juro.2008.03.045. dicussion 37. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Torres MP, Kaur S, et al. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene. 2015;34(16):2052–60. doi: 10.1038/onc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett’s esophagus. PloS ONE. 2013;8(1):e52192. doi: 10.1371/journal.pone.0052192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manjer J, Johansson R, Lenner P. Smoking is associated with postmenopausal breast cancer in women with high levels of estrogens. Int J Cancer. 2004;112(2):324–28. doi: 10.1002/ijc.20409. [DOI] [PubMed] [Google Scholar]
- 23.Coleman HG, Bhat S, Johnston BT, McManus D, Gavin AT, Murray LJ. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett’s esophagus. Gastroenterology. 2012;142(2):233–40. doi: 10.1053/j.gastro.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Han S, Yao Z, et al. A study of epidemiologic and recurrence factors of oral cancer. J Oral Maxillofac Surg. 2012;70(9):2205–10. doi: 10.1016/j.joms.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 25.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28(26):4052–57. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaupel P. Prognostic potential of the pre-therapeutic tumor oxygenation status. Adv Exp Med Biol. 2009;645:241–46. doi: 10.1007/978-0-387-85998-9_36. [DOI] [PubMed] [Google Scholar]
- 27.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 28.Kalra R, Singh SP, Pena-Philippides JC, Langley RJ, Razani-Boroujerdi S, Sopori ML. Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in an animal model. Clin Diagn Lab Immunol. 2004;11(3):563–68. doi: 10.1128/CDLI.11.3.563-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci USA. 2012;109(41):E2784–93. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu LM, Zavitz CC, Chen B, Kianpour S, Wan Y, Stampfli MR. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J Immunol. 2007;178(2):936–43. doi: 10.4049/jimmunol.178.2.936. [DOI] [PubMed] [Google Scholar]
- 32.Hilberink SR, Jacobs JE, van Opstal S, et al. Validation of smoking cessation self-reported by patients with chronic obstructive pulmonary disease. Int J Gen Med. 2011;4:85–90. doi: 10.2147/IJGM.S15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vartiainen E, Seppala T, Lillsunde P, Puska P. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health. 2002;56(3):167–70. doi: 10.1136/jech.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessides MC, Wheless L, Hoffman-Bolton J, Clipp S, Alani RM, Alberg AJ. Cigarette smoking and malignant melanoma: a case-control study. J Am Acad Dermatol. 2011;64(1):84–90. doi: 10.1016/j.jaad.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters EN, Torres E, Toll BA, et al. Tobacco assessment in actively accruing National Cancer Institute Cooperative Group Program Clinical Trials. J Clin Oncol. 2012;30(23):2869–75. doi: 10.1200/JCO.2011.40.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]


