ABSTRACT
A possible strategy to develop more diverse cell culture systems permissive to infection with naturally occurring prions is to exploit culture of neurospheres from transgenic mice expressing the normal prion protein (PrP) of the native host species. Accordingly, we developed differentiated neurosphere cultures from the cervid PrP-expressing mice to investigate whether this in vitro system would support replication of non-adapted cervid-origin chronic wasting disease (CWD) prions. Here we report the successful amplification of disease-associated PrP in differentiated neurosphere cultures within 3 weeks after exposure to CWD prions from both white-tailed deer or elk. This neurosphere culture system provides a new in vitro tool that can be used to assess non-adapted CWD prion propagation and transmission.
KEYWORDS: Chronic wasting disease, Neurosphere, Prion, Transgenic mice
INTRODUCTION
Prion diseases are fatal neurodegenerative disorders in humans and animals. These include Creutzfeldt-Jakob disease in humans, bovine spongiform encephalopathy in cattle, scrapie in sheep and goats, and chronic wasting disease (CWD) in cervids. Misfolding of the host-encoded cellular prion protein (PrPC) into its disease-associated form (PrPSc) is the hallmark of prion disease.1
To date, numerous cell cultures from different lineages are shown to be susceptible to prion infection.2 The prion-infected cell lines have been used as models to investigate mechanisms of prion propagation, seek therapeutic agents of prion diseases and understand the biology of PrPC. Only a limited number of cell lines have exhibited susceptibility to infection with non rodent-adopted prions.3-8 One possible strategy to develop more diverse cell culture systems permissive to infection with naturally occurring prions in humans and animals is the use of neural stem cells/neurospheres from transgenic (Tg) mouse lines expressing prion protein (PrP) of the native host species, since undifferentiated and differentiated neurospheres have been demonstrated to be permissive to mouse prion infection.9-12 Although several transgenic mice lines expressing PrPC from various species have been reported, no neurosphere model susceptible to infection with natural non-murine adapted prions has been reported.
In the present study, we isolated neurospheres from elk PrP expressing Tg5037 mice13 and demonstrated that differentiated neurospheres are susceptible to infection of non-adapted cervid-origin CWD prions as evidenced by the generation of proteinase-resistant PrPSc.
RESULTS
Neurospheres were cultured from the brains of neonatal Tg5037 mice expressing the elk PrP amino acid sequence (GenBank accession no.ABS87888.1) and in PrP-deficient (PrP0/0) mice harvested on the day of birth (identified as NP5037 and NP0, respectively), as described previously.12 The neurosphere cultures were passaged in serum-free media [Dulbecco's modified Eagle's medium-nutrient F12 Ham supplemented with N-2 factors, 50 ng/ml epidermal growth factor (EGF), 50 ng/ml basic fibroblast growth factor (bFGF) and penicillin and streptomycin] more than 8 times. The single-cell suspensions of neurospheres were cultured as monolayers in 6 well plates coated with fibronectin for 4 d. The cultures were differentiated by serum-free media supplemented with N-2 factor and any one of the following growth factors; i) EGF, ii) bFGF or iii) 5% fetal bovine serum (FBS). The differentiated cultures (termed as dNP0 and dNP5037 cultures) were harvested after 1 week post differentiation. The cell lysates of these cultures were then analyzed by western blotting using antibodies against nestin, a marker for potential neural stem cells; neuronal class III β-tubulin (Tuj1), a marker of early neuronal lineages; glial fibrillary acidic protein (GFAP), a marker for astrocyte lineage cells and PrP (Fig. 1A). The nestin levels were markedly decreased in the cultures differentiated by bFGF media (dNP/bFGF cultures). Under the all medium conditions, the levels of GFAP were markedly increased. The levels of Tuj1 were elevated in the cultures differentiated by EGF and FBS media (dNP/EGF and dNP/FBS, respectively). These results suggest that neurosphere cultures maintained the properties of self-renewal and multipotency. Phase-contrast microscopic images of the cultures illustrated that the cells in neurosphere cultures differentiated in EGF and bFGF media consisted mainly of small thin-bodied cells with long cellular process. On the other hand, the cells in cultures differentiated in FBS showed mainly flat sheet-like morphology with short cellular process (Fig. 1B).
FIGURE 1.
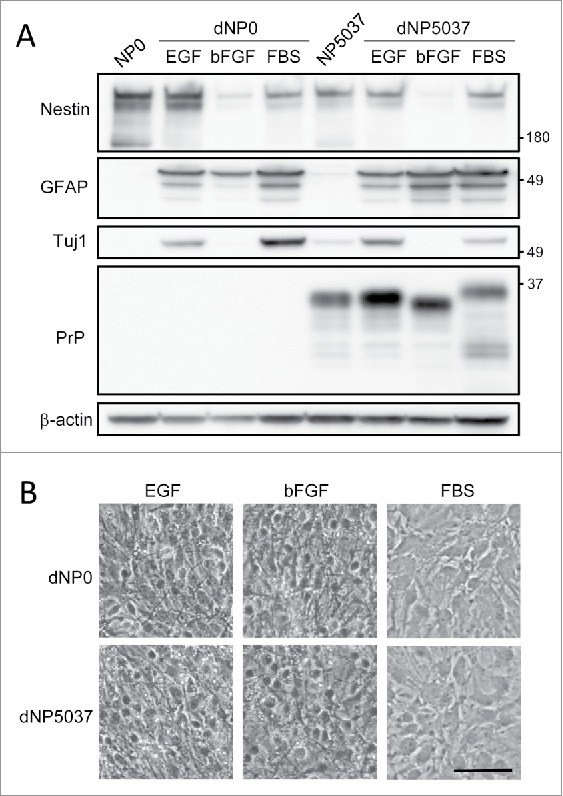
Neurospheres cultures differentiated under different culture conditions. Panel A. Western blot analysis of protein expression profiles. The neurosphere cultures from PrP-deficient mice and Tg5037 mice (NP0 and NP5037) were cultivated in monolayers and differentiated in the presence of any one of EGF, bFGF or FBS. The levels of PrP and marker proteins of differentiated cultures (dNP0 and dNP5037) were analyzed by western blotting using antibodies against nestin, a marker of potential neural stem cells, Tuj1, a marker of early neuronal lineage cells, GFAP, a marker of astrocyte lineage cells and PrP. Molecular mass standards (kDa) are indicated on the right. β -actin was used to normalize total protein levels. Panel B. Phase-contrast microscopic analysis of the cell morphology in dNP0 and dNP5037 cultures. Scale bar: 50 μm.
Next, we examined the susceptibility of dNP5037 cultures to CWD prion infection. At 4 d post differentiation, dNP0 and dNP5037 cultures were exposed to 2 ml of 0.01% (wt/vol) dilution of brain homogenates from CWD-positive white-tailed deer and incubated for 4 d. After exposure, culture medium was changed every 2 d. The accumulation of proteinase K (PK) resistant PrPSc (PrPres) in dNP5037 cultures was confirmed by western blot analysis at 3 weeks post infection (wpi). Of the 3 types of dNP5037 cultures differentiated in different media conditions, dNP5037/bFGF cultures accumulated substantial levels of PrPres (Fig. 2A). PrPres was also barely detectable in dNP5037/FBS cultures but not detectable in dNP5037/EGF cultures despite the appreciable expression of PrPC. CWD-infected dNP0 cultures, which served as nonPrPC expressing controls, remained negative. This suggests that the PrPres accumulation demonstrated in dNP5037/bFGF and FBS cultures was not due to residual PrPres that may have remained in the wells from the original inocula.
FIGURE 2.
Generation of PrPres in cervid PrP-expressing dNP5037/bFGF neurosphere cultures exposed to deer CWD. Panel A. Western blot analysis of total PrP and PrPres levels in cultures. dNP5037 and dNP0 cultures differentiated in the presence of indicated growth factors were exposed to 2 mL of 0.01% CWD infected (CWD/Deer) or normal (Mock) brain homogenates of white-tailed deer. The cultures were harvested at 3 weeks post infection (wpi). The levels of total PrP (upper panel) and PrPres (middle panel) in untreated and proteinase K (PK)-treated culture extracts were analyzed by western blotting using BAR224 Mab. Molecular mass standards (kDa) are indicated on the right. β-actin was used to normalize total protein levels. Panel B. Immunostaining of PrPSc of dNP5037 cultures at 3 wpi. Both dNP0 and dNP5037 cultures were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 and denaturized wit 5M guanidine thiocyanate. The cultures were immunolabeled with anti-PrP Mab132 and stained with AEC (3-amino-9-ethylcarbazole) substrate as chromogen (red). Nuclei were lightly counterstained with hematoxylin (blue). Scale bar: 100 μm.
PrPSc accumulation in dNP0/bFGF and dNP5037/bFGF cultures was also assessed by immunocytochemistry using MAb132.14 After guanidine thiocyanate treatment, CWD-infected dNP5037/bFGF cultures contained moderate levels of immunoreactivity at perinuclear sites whereas only faint staining was present in mock-infected dNP5037/bFGF cultures (Fig. 2B). No immunoreactivity was present in mock- and CWD-infected dNP0/bFGF cultures.
The previous study has shown that Tg5037 mice are susceptible to both CWD prions from white-tailed deer and elk.13 We exposed the dNP0 and dNP5037 cultures with CWD prions in brain from diseased elk and white-tailed deer and studied the kinetics of PrPres accumulation. Cultures were harvested at 1, 2 and 3 weeks post infection (wpi) and cell lysates treated with or without PK were analyzed by western blotting (Fig. 3). During the course of infection, gradual PrPres accumulation was observed in dNP5037/bFGF cultures infected with CWD prions from both elk and white-tailed deer and substantial levels of PrPres were detected at 3 wpi. By contrast, PrPres was barely detectable in dNP0 cells at 1 wpi and its levels gradually decreased thereafter. These results indicate de novo generation of PrPres in cervid PrPC expressing neuospheres, whereas PrP-deficient dNP0/bFGF cultures remained free of PrPres production.
FIGURE 3.
Kinetics of PrPres formation in cervid PrP-expressing dNP5037/bFGF neurosphere cultures. dNP5037/bFGF (cervid PrP) and dNP0/bFGF (PrP null) cultures were exposed to 2 mL of 0.01% CWD infected or normal (Mock) brain homogenates of elk (Panel A, CWD/Elk) or white-tailed deer (Panel B, CWD/Deer), cultured and harvested 1, 2 and 3 weeks post infection (wpi). The levels of total PrP (upper of each panel) and PrPres (middle of each panel) in untreated and PK-treated culture extracts were analyzed by western blotting using BAR224 Mab. Molecular mass standards (kDa) are indicated on the right. β-actin was used to normalize total protein levels.
To date, 2 cell lines have been developed that are susceptible to infection with CWD.3,5 Cervid prion cell assay using rabbit kidney epithelial (RK13) cells expressing elk PrP generates PrPres foci after exposure to as little as 0.1 μg of elk CWD brain tissue.5 To evaluate the sensitivity of the model, dNP5037/bFGF cultures were exposed to 2 ml of serially 10-fold diluted 0.01% (wt/vol) CWD white-tailed deer brain homogenates (200-0.02 μg brain tissue equivalent). In parallel, to monitor the levels of residual input PrPSc, dNP0/bFGF cultures were exposed to the same inocula. The accumulation of PrPres in these cultures was assessed by western blotting at 4 wpi. PrPres was detected in dNP5037/bFGF cultures exposed to as little as 2 μg of CWD positive deer brain (Fig. 4). Conversely, PrPres was not detectable in dNP0/bFGF cultures exposed to CWD brain tissue.
FIGURE 4.
Sensitivity of cervid PrP dNP5037/bFGF cultures. dNP5037/bFGF and PrP null dNP0/bFGF cultures were exposed to 2 mL of serial 10-fold dilutions of 0.01% CWD infected (CWD/Deer, 200 to 0.2 μg brain tissue equivalent) or normal (Mock, 200 μg) white-tailed deer brain homogenates (BH), cultured and harvested 4 weeks post infection. The levels of total PrP (upper panel) in untreated culture extracts and PrPres (middle panel) in PK-treated culture extracts were analyzed by western blotting using BAR224 Mab. Molecular mass standards (kDa) are indicated on the right. β-actin was used to normalize total protein levels.
DISCUSSION
In this study, we demonstrated that differentiated neurospheres from elk PrP-overexpressing mice were susceptible to infection with non-adapted CWD prions sourced from white-tailed deer and elk.
Of the 3 types of dNP5037 cultures differentiated in various media conditions, only dNP5037/bFGF cultures accumulated the substantial levels of PrPres after exposure to CWD prions. We observed differences in molecular weights of the di-glycosylated PrPC from these differentiated cultures (Fig. 1A), possibly reflecting differences in the constituent cell types generated by each growth factors. Thus it is conceivable that the susceptible cell types capable of efficiently replicating CWD prions may be represented more abundantly in dNP5037/bFGF cultures.
We previously reported that the neurosphere cultures from transgenic mice expressing murine PrP were susceptible to prion infection after differentiation by serum-free media supplemented with N-2 factor and EGF (dNP20/EGF cultures).12 In the current study, despite applying the same differentiation condition as dNP20/EGF cultures, the dNP5037/EGF cultures were not susceptible to prion infection. The inconsistency in prion susceptibility between mouse PrP dNP20/EGF and cervid PrP dNP5037/EGF cultures could be related to the different transgenic mouse lines (Tga20 vs Tg5037) used for these studies, as it is known that transgene expression is influenced by genome insertion position and multipllcity. Our results suggest that before prion infection, the individualized optimization of differentiation condition is necessary for neurosphere cultures form each Tg mice lines.
In summary, differentiated neurospheres from Tg5037 mice expressing cervid PrP represent a new in vitro tool that can be used to assess non-adapted CWD prion propagation and transmission.
MATERIALS AND METHODS
Anti-neuronal class III β-tubulin (Tuj1) monoclonal antibody(Mab) was obtained from Covance (Princeton, NJ). Anti-glial fibrillary acidic protein (GFAP) Mab and Anti-nestin Mab were obtained from Cell Signaling Technology (Danvers, MA). Anti-b-actin was obtained from Sigma (St. Louis, MO). Anti-PrP Mab BAR224 was obtained from Cayman Chemical Company (Ann Arbor, MI). Anti-PrP Mab13214 was donated by M. Horiuchi (Hokkaido University, Japan).
All animals were handled in strict accordance with guidelines for animal care and use provided by the United States Department of Agriculture (USDA), National Institutes of Health (NIH) and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), and all animal work was approved by Colorado State University Institutional Animal Care and Use Committee (IACUC) Institutional Animal Care and Use Committee (IACUC) (approval numbers 02–151A, 08–175A and 11–2615A).
Neurospheres were cultured from the neonatal brains of Tg5037 mice13 and PrP-deficient mice at day 0 after birth.Cortices were minced by sterilized disposable micro homogenizer (biomasher, Nippi, Tokyo, Japan) and incubated in a solution of proteolytic and collagenolytic enzymes (Accutase, Innovative Cell Technologies, San Diego, CA) supplemented with 400 μg/ml DNase I for 15 min at 37 °C with constant agitation. The cell suspensions were prepared by repeated pipetting using aerosol resistant tip and placed into 15-ml centrifuge tubes for 2 min. Cell suspensions without tissue fragments were collected and centrifuged at 400 × g for 5 min. Cells were suspended in Dulbecco's Modified Eagle's Medium/Nutrient F-12 Ham (Sigma) supplemented with N-2 factors (N-2 supplement, Life Technologies Corporation, Carlsbad, CA), 50 ng/ml epidermal growth factor (EGF, Sigma), 50 ng/ml basic fibroblast growth factor (bFGF, Sigma), and penicillin and streptomycin (Sigma) and recentrifuged at 400 × g for 5 min. Cells were resuspended in culture medium and plated in 60-mm culture dishes having low adherability (HydroCell, CellSeed, Tokyo, Japan). Cultures were maintained at 37 °C with 5% CO2 as floating spheres with daily medium addition for 4 or 5 d. Spheres were dissociated in Accutase at 37 °C for 10 min with trituration and passed at 1:4 every 3-4 d.
For monolayer cultures, 6 well culture plate (Primaria, Corning, Corning, NY) were incubated overnight with 12.5 μg/ml fibronectin (Sigma) at 37 °C in a humidified incubator and desiccated for 2 h at room temperature. Single-cell suspensions were prepared by Accutase treatment with trituration and plated at 200,000 cells per on fibronectin-coated 6-well plates for 3-4 d upon reaching 90% confluence. Differentiation was induced by withdrawal of i) EGF ii) bFGF iii) both bFGF and EGF with addition 5% fetal bovine serum. Culture medium was replaced every 2 d. At day 4 after differentiation, cultures were treated with various concentrations of brain homogenates from CWD positive white-tailed deer15 or elk (homogenized in PBS, 10% w/v stock) diluted in 2 ml of culture medium and incubated. After 48 h, fresh culture medium was added and the cultures were incubated for another 48 h. Culture medium was changed every 2 d.
The cultures were washed with PBS and subjected to 3 freeze-thaw cycles in 500 uL PBS. Freeze-thaw lysates were stored for western blotting (WB). Whole cell extracts were prepared with lysis buffer containing 1 mM EDTA with 0.5% Triton X-100, 0.5% sodium deoxycholate, and protease inhibitors (Complete, Roche Diagnostics, Basel, Switzerland) in PBS. After the removal of insoluble debris by centrifugation at 10,000 × g for 2 min, the total protein concentrations of the samples were measured by the bicinchoninic acid assay (BCA protein assay, Thermo Fisher Scientific, Rockland, IL), and equal amounts of protein were analyzed. For the detection of proteinase resistant PrPSc (PrPres), samples were digested 20 μg/mL proteinase K for 15 min at 37 °C. The reactions were stopped by the addition of 4 mM Pefabloc (Roche Diagnostics). Cell lysates were then ultracentrifuged at 200,000 × g for 1 h in a TLA 55 rotor (Beckman Coulter, Fullerton, CA) and the resulting pellets were used for further analysis. The samples were solubilized in LDS-sample loading buffer (Invitrogen, San Diego, CA) and boiled. The samples were electrophoresed on 12 NOVEX pre-cast gels (Life Technologies Corporation), electrotransfered onto Durapore (Millipore) polyvinylidene fluoride membranes and probed with. ECL Prime (GE Healthcare Life Sciences, Buckinghamshire, UK) was used for immunodetection. The blots were imaged with a ImageQuant LAS4000 (GE Healthcare) and analyzed using image reader software (αEaseFC; α Innotech, San Leandro, CA) according to the manufacturer's instructions.
For immunocytochemistry, the cultured cells were rinsed with PBS, fixed with 4% paraformaldehyde in PBS for 30 min at room temperature, treated with 0.5% Triton X-100 in PBS, incubated with 200 mM glycine in PBS for 5 min, then treated with 5 M guanidine thiocyanate for 10 min at room temperature and rinsed thrice with PBS for 5 min. After incubation with blocking solution (1% bovine serum albumin, 1% normal goat serum, and 0.05% Tween 20 in PBS) for 30 min, the cells were labeled with Mab132 in blocking solution for 1 h at room temperature. After 3 washes with 0.05% Tween 20 in PBS, cells were then further incubated Envision+ anti-mouse HRP labeled polymer (Dako, Glostrup, Denmark) and incubated with AEC Substrate-Chromagen (Dako) before counterstain with hematoxylin and reading by light microscopy.
ABBREVIATIONS
- bFGF
basic fibroblast growth factor
- CWD
chronic wasting disease
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- PK
proteinase K
- PrP
prion protein
- PrPres
proteinase K resistant disease-associated prion protein
- PrPSc
disease-associated prion protein
- PrPC
cellular prion protein
- Tg
transgenic
- wpi
weeks post infection
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We thank Jeanette Hayes-Klug, \Kelly Anderson, Erin McNulty, Kim Sehun and Stephanie Fullaway at Colorado State University for their tireless efforts in animal care and welfare.
FUNDING
This work was supported by grant 1-R01-NS061902 from the US National Institutes of Health and by the National Agriculture and Food Research Organization of Japan under a research fellowship.
REFERENCES
- [1].Prusiner SB. Molecular biology of prion diseases. Science 1991; 252(5012):1515-22; PMID:1675487; https://doi.org/ 10.1126/science.1675487 [DOI] [PubMed] [Google Scholar]
- [2].Grassmann A, Wolf H, Hofmann J, Graham J, Vorberg I. Cellular aspects of prion replication in vitro. Viruses 2013; 5(1):374-405; PMID:23340381; https://doi.org/ 10.3390/v5010374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raymond G, Olsen E, Lee K, Raymond L, Bryant Pr, Baron G, Caughey W, Kocisko D, McHolland L, Favara C, et al.. Inhibition of protease-resistant prion protein formation in a transformed deer cell line infected with chronic wasting disease. J Virol 2006; 80(2):596-604; PMID:16378962; https://doi.org/ 10.1128/JVI.80.2.596-604.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neale M, Mountjoy S, Edwards J, Vilette D, Laude H, Windl O, Saunders G. Infection of cell lines with experimental and natural ovine scrapie agents. J Virol 2010; 84(5):2444-52; PMID:20032176; https://doi.org/ 10.1128/JVI.01855-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bian J, Napier D, Khaychuck V, Angers R, Graham C, Telling G. Cell-based quantification of chronic wasting disease prions. J Virol 2010; 84(16):8322-6; PMID:20519392; https://doi.org/ 10.1128/JVI.00633-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vilette D, Andreoletti O, Archer F, Madelaine M, Vilotte J, Lehmann S, Laude H. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc Natl Acad Sci U S A 2001; 98(7):4055-9; PMID:11259656; https://doi.org/ 10.1073/pnas.0613379\98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Archer F, Bachelin C, Andreoletti O, Besnard N, Perrot G, Langevin C, Le Dur A, Vilette D, Baron-Van Evercooren A, Vilotte J, et al.. Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J Virol 2004; 78(1):482-90; PMID:14671128; https://doi.org/ 10.1128/JVI.78.1.482-490.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tark D, Kim H, Neale MH, Kim M, Sohn H, Lee Y, Cho I, Joo Y, Windl O. Generation of a persistently infected MDBK cell line with natural bovine spongiform encephalopathy (BSE). PLoS One 2015; 10(2):e0115939; PMID:25647616; https://doi.org/ 10.1371/journal.pone.0115939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Giri R, Young R, Pitstick R, DeArmond S, Prusiner S, Carlson G. Prion infection of mouse neurospheres. Proc Natl Acad Sci U S A 2006; 103(10):3875-80; PMID:16495413; https://doi.org/ 10.1073/pnas.0510902103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Milhavet O, Casanova D, Chevallier N, McKay R, Lehmann S. Neural stem cell model for prion propagation. Stem Cells 2006; 24(10):2284-91; PMID:16741225; https://doi.org/ 10.1634/stemcells.2006-0088 [DOI] [PubMed] [Google Scholar]
- [11].Herva M, Relaño-Ginés A, Villa A, Torres J. Prion infection of differentiated neurospheres. J Neurosci Methods 2010; 188(2):270-5; PMID:20206206; https://doi.org/ 10.1016/j.jneumeth.2010.02.022 [DOI] [PubMed] [Google Scholar]
- [12].Iwamaru Y, Takenouchi T, Imamura M, Shimizu Y, Miyazawa K, Mohri S, Yokoyama T, Kitani H. Prion replication elicits cytopathic changes in differentiated neurosphere cultures. J Virol 2013; 87(15):8745-55; PMID:23740992; https://doi.org/ 10.1128/JVI.00572-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Angers R, Seward T, Napier D, Green M, Hoover E, Spraker T, O'Rourke K, Balachandran A, Telling G. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis 2009; 15(5):696-703; PMID:19402954; https://doi.org/ 10.3201/eid1505.081458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamasaki T, Suzuki A, Shimizu T, Watarai M, Hasebe R, Horiuchi M. Characterization of intracellular localization of PrP(Sc) in prion-infected cells using a mAb that recognizes the region consisting of aa 119–127 of mouse PrP. J Gen Virol 2012; 93(Pt 3):668-80; PMID:22090211; https://doi.org/ 10.1099/vir.0.037101-0 [DOI] [PubMed] [Google Scholar]
- [15].Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One 2013; 8(11):e80203; PMID:24224043; https://doi.org/ 10.1371/journal.pone.0080203 [DOI] [PMC free article] [PubMed] [Google Scholar]


