Abstract
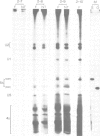
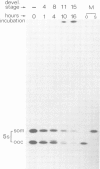
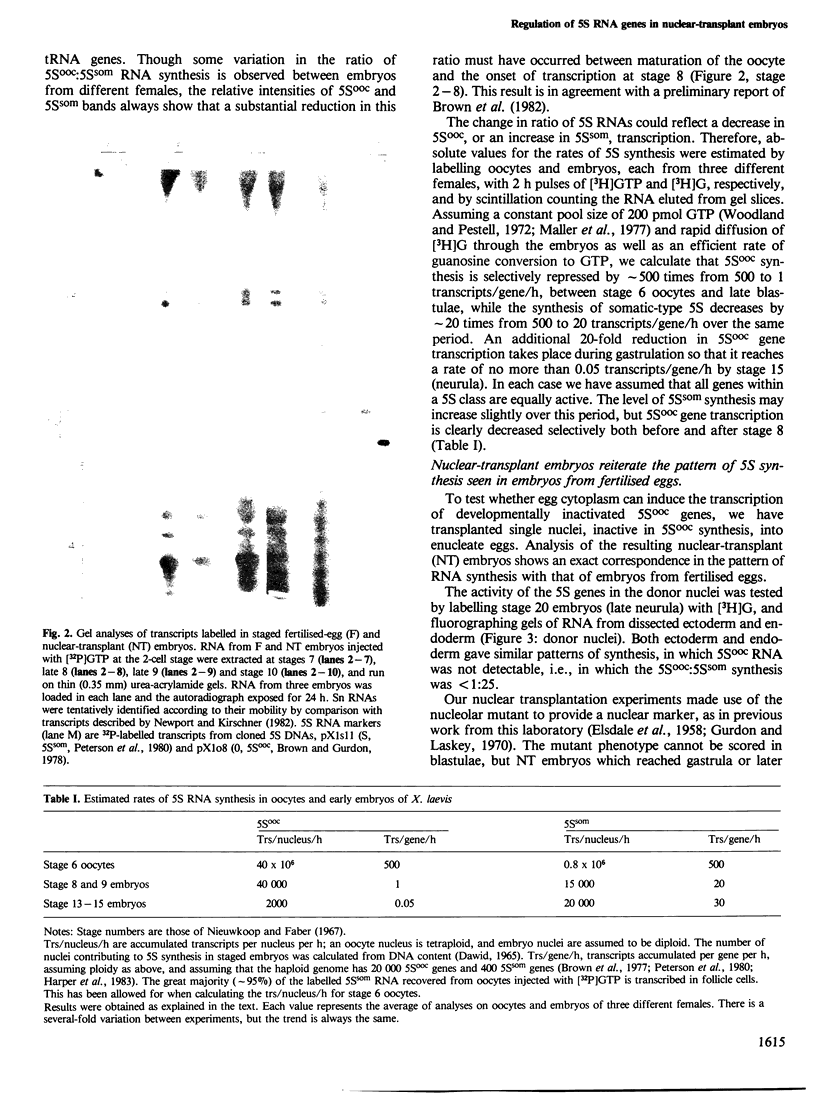
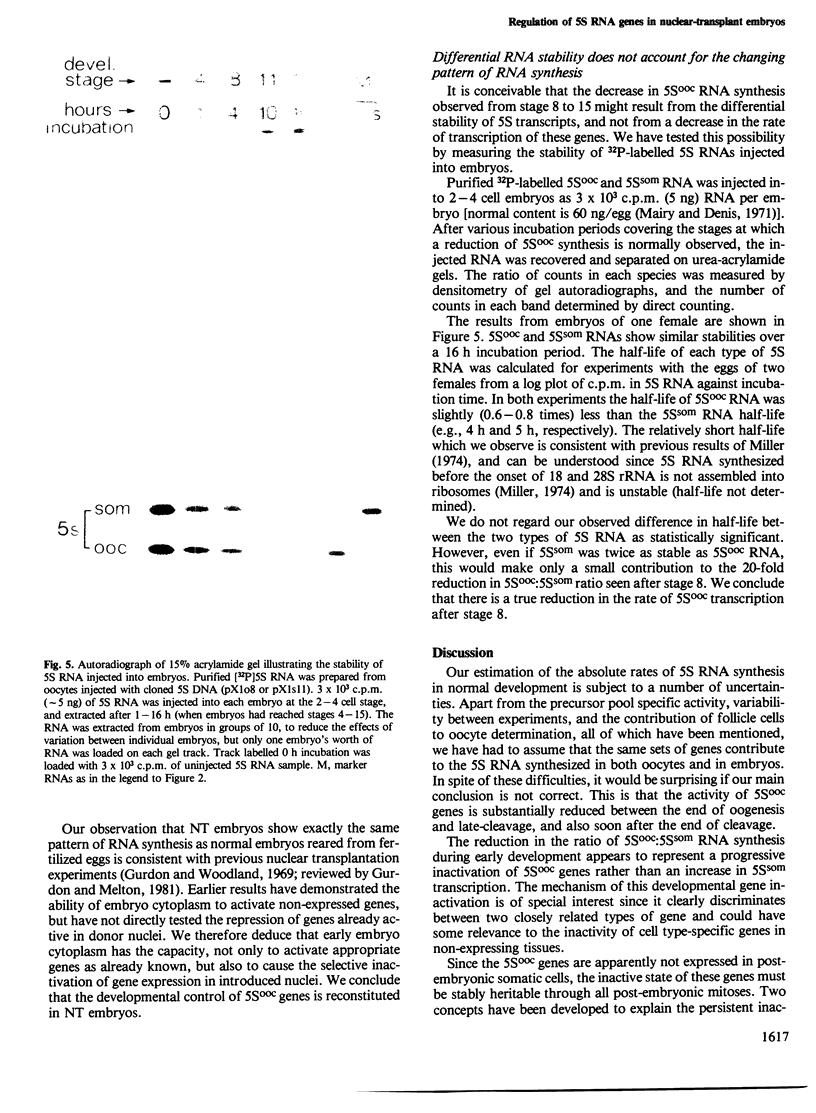
In the normal development of Xenopus laevis, genes for oocyte-type and somatic-type 5S RNAs are both expressed in late blastulae. Estimates of rates of synthesis indicate that the oocyte-type genes (5Sooc) undergo at least a 100-fold reduction in transcriptional activity between the end of oogenesis and the late blastula stage, and at least a further 20-fold reduction during gastrulation. When neurula nuclei, with inactive 5Sooc genes, were transplanted to enucleated eggs, the resulting nuclear-transplant embryos showed a reactivation of 5Sooc genes in blastulae and a subsequent inactivation after this stage. This effect is not explicable by a differential stability of the two types of 5S RNA. We conclude that egg or early embryo cytoplasm must contain components which can continuously regulate 5S gene expression, and that the mechanism by which 5Sooc genes are developmentally inactivated does not persist through mitosis in early embryos. These results have been obtained by a new procedure in which oocyte- and somatic-type 5S RNAs are separated in a 4 M urea-15% acrylamide gel.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachvarova R., Davidson E. H., Allfrey V. G., Mirsky A. E. Activation of RNA synthesis associated with gastrulation. Proc Natl Acad Sci U S A. 1966 Feb;55(2):358–365. doi: 10.1073/pnas.55.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D. F., Wormington W. M., Brown D. D. Stable transcription complexes of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982 Feb;28(2):413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Carrol D., Brown R. D. The isolation and characterization of a second oocyte 5s DNA from Xenopus laevis. Cell. 1977 Dec;12(4):1045–1056. doi: 10.1016/0092-8674(77)90168-4. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Gurdon J. B. Cloned single repeating units of 5S DNA direct accurate transcription of 5S RNA when injected into Xenopus oocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2849–2853. doi: 10.1073/pnas.75.6.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B. Deoxyribonucleic acid in amphibian eggs. J Mol Biol. 1965 Jul;12(3):581–599. doi: 10.1016/s0022-2836(65)80313-8. [DOI] [PubMed] [Google Scholar]
- ELSDALE T. R., FISCHBERG M., SMITH S. A mutation that reduces nucleolar number in Xenopus laevis. Exp Cell Res. 1958 Jun;14(3):642–643. doi: 10.1016/0014-4827(58)90175-7. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Ford P. J., Brown R. D. Sequences of 5S ribosomal RNA from Xenopus mulleri and the evolution of 5S gene-coding sequences. Cell. 1976 Aug;8(4):485–493. doi: 10.1016/0092-8674(76)90216-6. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- GURDON J. B. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J Embryol Exp Morphol. 1960 Dec;8:505–526. [PubMed] [Google Scholar]
- Gottesfeld J., Bloomer L. S. Assembly of transcriptionally active 5S RNA gene chromatin in vitro. Cell. 1982 Apr;28(4):781–791. doi: 10.1016/0092-8674(82)90057-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Laskey R. A. The transplantation of nuclei from single cultured cells into enucleate frogs' eggs. J Embryol Exp Morphol. 1970 Sep;24(2):227–248. [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Methods for nuclear transplantation in amphibia. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Woodland H. R. The influence of the cytoplasm on the nucleus during cell differentiation, with special reference to RNA synthesis during amphibian cleavage. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):99–111. doi: 10.1098/rspb.1969.0042. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Price J., Korn L. J. Chromosomal mapping of Xenopus 5S genes: somatic-type versus oocyte-type. Nucleic Acids Res. 1983 Apr 25;11(8):2313–2323. doi: 10.1093/nar/11.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Gurdon J. B., Price J. Oocyte extracts reactivate developmentally inert Xenopus 5S genes in somatic nuclei. Nature. 1982 Nov 25;300(5890):354–355. doi: 10.1038/300354a0. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Gurdon J. B. The reactivation of developmentally inert 5S genes in somatic nuclei injected into Xenopus oocytes. Nature. 1981 Feb 5;289(5797):461–465. doi: 10.1038/289461a0. [DOI] [PubMed] [Google Scholar]
- Korn L. J. Transcription of Xenopus 5S ribosomal RNA genes. Nature. 1982 Jan 14;295(5845):101–105. doi: 10.1038/295101a0. [DOI] [PubMed] [Google Scholar]
- Mairy M., Denis H. Recherches biochimiques sur l'oogenèse. I. Synthèse et accumulation du RNA pendant l'oogenèse du crapaud sud-africain Xenopus laevis. Dev Biol. 1971 Feb;24(2):143–165. doi: 10.1016/0012-1606(71)90092-3. [DOI] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. Metabolism of 5S RNA in the absence of ribosome production. Cell. 1974 Nov;3(3):275–281. doi: 10.1016/0092-8674(74)90142-1. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Wormington W. M., Brown D. D. Related 5S RNA transcription factors in Xenopus oocytes and somatic cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1760–1764. doi: 10.1073/pnas.78.3.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. C., Doering J. L., Brown D. D. Characterization of two xenopus somatic 5S DNAs and one minor oocyte-specific 5S DNA. Cell. 1980 May;20(1):131–141. doi: 10.1016/0092-8674(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Reynolds W. F., Bloomer L. S., Gottesfeld J. M. Control of 5S RNA transcription in Xenopus somatic cell chromatin: activation with an oocyte extract. Nucleic Acids Res. 1983 Jan 11;11(1):57–75. doi: 10.1093/nar/11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland H. R., Gurdon J. B. The relative rates of synthesis of DNA, sRNA and rRNA in the endodermal region and other parts of Xenopus laevis embryos. J Embryol Exp Morphol. 1968 May;19(3):363–385. [PubMed] [Google Scholar]
- Woodland H. R., Pestell R. Q. Determination of the nucleoside triphosphate contents of eggs and oocytes of Xenopus laevis. Biochem J. 1972 Apr;127(3):597–605. doi: 10.1042/bj1270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M., Bogenhagen D. F., Jordan E., Brown D. D. A quantitative assay for Xenopus 5S RNA gene transcription in vitro. Cell. 1981 Jun;24(3):809–817. doi: 10.1016/0092-8674(81)90106-9. [DOI] [PubMed] [Google Scholar]
- Young D., Carroll D. Regular arrangement of nucleosomes on 5S rRNA genes in Xenopus laevis. Mol Cell Biol. 1983 Apr;3(4):720–730. doi: 10.1128/mcb.3.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]