Abstract
Oxytocin (OT) is a hypothalamic neuropeptide that modulates fear and anxiety-like behaviors. Dorsolateral bed nucleus of the stria terminalis (BNSTdl) plays a critical role in the regulation of fear and anxiety, and expresses high levels of OT receptor (OTR). However, the role of OTR neurotransmission within the BNSTdl in mediating these behaviors is unknown. Here, we used adult male Sprague-Dawley rats to investigate the role of OTR neurotransmission in the BNSTdl in the modulation of the acoustic startle response, as well as in the acquisition and consolidation of conditioned fear using fear potentiated startle (FPS) paradigm. Bilateral intra-BNSTdl administration of OT (100 ng) did not affect the acquisition of conditioned fear response. However, intra-BNSTdl administration of specific OTR antagonist (OTA), (d(CH2)51, Tyr(Me)2, Thr4, Orn8, des-Gly-NH29)-vasotocin, (200 ng), prior to the fear conditioning session, impaired the acquisition of cued fear, without affecting a non-cued fear component of FPS. Neither OTA, nor OT affected baseline startle or shock reactivity during fear conditioning. Therefore, the observed impairment of cued fear after OTA infusion resulted from the specific effect on the formation of cued fear. In contrast to the acquisition, neither OTA nor OT affected the consolidation of FPS, when administered after the completion of fear conditioning session. Taken together, these results reveal the important role of OTR neurotransmission in the BNSTdl in the formation of conditioned fear to a discrete cue. This study highlights the role of the BNSTdl in learning to discriminate between threatening and safe stimuli.
Keywords: oxytocin, fear, anxiety, startle, learning, BNST
1. Introduction
Oxytocin (OT) is a hypothalamic neuropeptide that modulates a wide range of social behaviors, as well as fear and anxiety-like behaviors, for review see (Neumann and Slattery, 2016). Although substantial evidence suggests that OT has anxiolytic properties (Bale et al., 2001; Ellenbogen et al., 2014; Ring et al., 2006), the role of OT neurotransmission in the regulation of conditioned fear appears to be more complex and brain site-specific. When applied intracerebroventricularly (ICV) prior to the fear conditioning, OT reduces cued fear expression, but OT impairs cued fear extinction when delivered prior to the extinction training (Toth et al., 2012). In addition, OT in the central amygdala (CeA) (Knobloch et al., 2012) and medial prefrontal cortex (Lahoud and Maroun, 2013) reduces contextual fear expression, but the opposite effect is observed when OT is delivered into the basolateral amygdala (Lahoud and Maroun, 2013), or when OT receptors (OTR) are overexpressed in the lateral septum (Guzman et al., 2013). In the fear potentiated startle (FPS) experiments systemic OT decreases background anxiety, but it has no effect on cued or contextual fear, when administered systemically or ICV (Ayers et al., 2011; Missig et al., 2010). In these experiments, cued fear was measured as a potentiation of the startle amplitude that occurred in a presence of conditioned stimulus (CS+). Background anxiety was expressed as a potentiation of the startle amplitude during noise-alone trials (CS−) that occurred after the first presentation of CS+ (measured in a novel context). Contextual fear was measured as a startle potentiation to noise-only trials (no CS presentation) in the training context.
Dorso-lateral bed nucleus of the stria terminalis (BNSTdl) is a key brain area translating stress into sustained anxiety (Dabrowska et al., 2013; Daniel and Rainnie, 2016; Davis et al., 2010; Sparta et al., 2013). While involvement of the BNST in the light- (Walker and Davis, 1997) or corticotropin-releasing factor (CRF) potentiated startle (Lee and Davis, 1997) is well documented, the role of the BNST in the modulation of cued fear is less apparent. BNST lesions disrupt expression of contextual fear (Sullivan et al., 2004), as well as conditioned fear response to long-lasting cues (Davis et al., 2010), but not short, discrete cues (Gewirtz et al., 1998; Hitchcock and Davis, 1991; LeDoux et al., 1988). However, most of the initial reports stem from lesion studies, whereas accumulating evidence from cell-type specific manipulations supports the notion that the BNST might also be involved in the conditioned fear response to discrete cues, for review see (Gungor and Pare, 2016). The BNST has one of the highest expression levels of OTR (Dabrowska et al., 2011; Dumais et al., 2013; Tribollet et al., 1988; Veinante and Freund-Mercier, 1997) and receives dense OT inputs from the paraventricular nucleus of the hypothalamus (Dabrowska et al., 2011; Knobloch et al., 2012), yet the role of OTR neurotransmission in the BNST in the regulation of fear and anxiety is unknown.
Here, we examined the effects of OT and OTR antagonist administration into the BNSTdl on the acoustic startle response (ASR), as well as on the acquisition and consolidation of cued and non-cued fear using FPS paradigm. Using in vivo pharmacological approach we demonstrate for the first time that OTR neurotransmission in the BNSTdl facilitates the acquisition, but not consolidation, of conditioned fear to a discrete cue.
2. Material and methods
2.1 Animals
Adult male Sprague-Dawley rats aged 44–48 days old and weighing 175–199 g were purchased from ENVIGO (IL). The rats were housed in groups of three on a 12 h light/dark cycle (light 7 a.m. to 7 p.m.) with free access to water and food. The rats were allowed to adapt to this environment for one week before the experiments began. A total of 97 animals were used in these experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committees at Rosalind Franklin University of Medicine and Science, and were performed in accordance with the US National Institutes of Health guidelines.
2.2 Drugs
OT (H-2510, Bachem Inc., CA) and the selective OTR antagonist (OTA, H-2908, Bachem Inc., CA), (d(CH2)51, Tyr(Me), Thr4, Orn8, des-Gly-NH29)-vasotocin (Manning et al., 2012) were stored in −80 Celsius degrees freezer and diluted in artificial cerebrospinal fluid (ACSF, pH = 7.4) before an experiment.
2.3 Surgery
Rats weighing between 230–270 g were deeply anesthetized with mix of isoflurane and oxygen and placed in a stereotaxic frame (Model 900; Kopf, CA). Ketoprofen was used as an analgesic (5 mg kg−1, subcutaneous; Zoetis Inc., MI). Rats were bilaterally implanted with guide cannula (22-gauge, 7 mm length; Plastics One, Roanoke, VA) aimed at the BNSTdl using the following stereotaxic coordinates (15° coronal angle, from bregma, AP: +0.1 mm; ML: ±3.4 mm; DV: −5.25 mm). Stereotaxic coordinates were based on the rat brain atlas (Paxinos and Watson, 2007) and adapted from the BNSTdl coordinates we have published before (Dabrowska et al., 2016). Guide cannulae were positioned 2 mm above the BNSTdl and fixed with dental cement bonded to stainless steel screws inserted into the surface of the skull. A dummy cap (7 mm length; Plastics One, Roanoke, VA) was inserted into the guide cannula to maintain it free of obstructions. Following surgery, the rats were allowed to recover for 4–7 days, during which they were handled and their cannulas checked daily to habituate them to the injection procedure.
2.4 Drug administration
OT (100 ng), OTA (200 ng), or ACSF (all in volume of 0.5 μl per side) were injected bilaterally into the BNSTdl through microinjector (28-gauge, 7 mm length; Plastics One, Roanoke, VA). Injections were made at a rate of 0.25 μl min−1 using microinjection pump (Harvard Apparatus, MA), connected via polyethylene tubing (PE-20) to Hamilton syringes. After the injection, the microinjector was left in place for additional 5 min for adequate diffusion. The doses of OT and OTA were chosen based on previous studies on fear and anxiety in rats (Bale et al., 2001; Lahoud and Maroun, 2013; Neumann and Slattery, 2016; Neumann et al., 2000; Toth et al., 2012).
2.5 Acoustic startle apparatus
All experiments were conducted in eight, identical SR-LAB startle chambers with cylindrical animal enclosures (San Diego Instruments, San Diego, CA). A high-frequency loudspeaker, mounted 24 cm above the enclosures, provides background noise as well as the startle eliciting white-noise bursts (WNB). During the FPS, a single LED bulb positioned on the ceiling inside the startle chamber was used as the visual conditioned stimulus (CS). In addition, a grid floor made of stainless steel bars placed inside the enclosures was used to deliver foot shocks as the unconditioned stimulus (US). The presentation and sequence of all stimuli as well as recording of the responses were automatically controlled by the SR-LAB software (San Diego Instruments).
2.6 Acoustic startle response (ASR)
On day 1 (habituation), rats were placed in the cylindrical enclosures inside the chambers for 20 min. On day 2 (pre-test), rats were placed in the same enclosures, where after 5 min acclimation they were presented with 30 startle eliciting WNB (95 dB, 50 ms, inter-trial-interval 30 s). A background white-noise (70 dB) was continuously played throughout the whole session. On day 3 (test), rats received 30-startle eliciting WNB (same as above) 10 min after bilateral administration of OT (n=7), OTA (n=7) or ACSF (n=7) into the BNSTdl (Fig. 1).
Fig. 1.

Schematic representation of the experimental design. In the acoustic startle response (ASR) experiments, rats were given intra-BNSTdl ACSF (0.5 μl in each side), OT (100 ng in 0.5 μl) or OTA (200 ng in 0.5 μl) 10 min prior to the test session. In the fear potentiated startle (FPS) experiments, rats were given intra-BNSTdl ACSF, OT or OTA (doses as above) either 10 min prior to the fear conditioning session (acquisition) or 30 min following the completion of the fear conditioning session (consolidation). FPS expression (measured as cued and non-cued fear, was tested 24 hours later. Cue light, conditioned stimulus (CS, 3.7 s); foot shock, unconditioned stimulus (US, 0.5 s, 0.5 mA); white-noise burst (WNB).
In all experiments, animals were assigned to the experimental groups based on their baseline ASR (pre-test) in order to generate experimental groups with balanced average ASR and to reduce variability in stress-reactivity. All chambers were cleaned with a 70% ethanol solution between sessions.
2.7 Fear potentiated startle (FPS)
The FPS procedures were modified based on previous studies (Ayers et al., 2011; Missig et al., 2010; Sink et al., 2013; Walker et al., 2009a). On days 1 and 2, separate cohort of rats underwent habituation and pre-test sessions, respectively (as above). On the following day (fear conditioning), animals were placed in the cylindrical enclosures containing a grid floor conveying foot shocks. After 5 min acclimation, animals received 10 presentations of a 3.7 s cue light (CS), each co-terminating with a 0.5 s foot shock (US; 0.5 mA, inter-trial-interval 60–180 s). A background noise was absent during the conditioning session. Twenty-four hours later, rats were tested for the FPS expression (test), where after 5 min acclimation they were exposed to 30 startle eliciting WNB (as above) and their levels of cued and non-cued fear, were measured. A background white-noise of 70 dB was continuously played throughout the whole session. The session consisted of 10 baseline startle trials (not included in the analysis) followed by additional 20 trials, with half presented in the presence of the cue light (CS) and the other half without the CS (noise-alone), mixed in a pseudorandom order (inter-trial-interval 30 s). During the FPS testing, grid floor was removed from the enclosures.
To examine the role of OT in the acquisition of fear conditioning, animals were divided based on pre-test ASR into three treatment groups: OT (n=13), OTA (n=20), or ACSF (n=21). Fear conditioning session was performed 10 min after the intra-BNSTdl injections (Fig. 1).
Another cohort of rats was used for the FPS consolidation experiment. Here, rats received OT (n=7), OTA (n=7), or ACSF (n=8), 30 min after completing the fear conditioning session (Fig. 1).
2.8 Histology
Following the experiments, rats were bilaterally injected with 1% Chicago Sky Blue dye (Alfa Aesar, MA) into the BNSTdl (0.5 μl). The brains were removed and coronal sections (50 μm) were cut on a Leica freezing-stage sliding microtome (SM200R, Leica Biosystems Inc., IL) and photographed to assess proper cannula placement (Fig. 2). Data from rats with guide cannula placement outside the BNSTdl, including posterior, anterior, or sub-commissural BNST were excluded from the analyses. Rats with both unilateral and bilateral hits were included in the analysis.
Fig. 2.

Proper cannula placement was verified with bilateral injection of 1% Chicago Sky Blue dye into the BNSTdl (dark arrows). Cued and non-cued fear were measured during FPS test session and calculated as percent change scores of startle amplitude.
2.9 Data analysis
Startle amplitude was defined as the maximum peak voltage within the first 200 ms after the onset of WNB. Shock reactivity amplitude was recorded during the fear conditioning session and was defined as the maximum peak voltage that occurred during the 0.5 s foot shock delivery. Cued and non-cued fear were calculated as percent change scores of startle amplitude based on (Ayers et al., 2011; Missig et al., 2010; Walker et al., 2009a). Cued fear = [(light-noise trials − noise-alone trials)/noise-alone trials] × 100. Non-cued fear = [(noise-alone trials − pre-shock trials)/pre-shock trials] × 100 (Fig. 2).
2.10 Statistical analysis
All data are presented as mean ± standard error of mean (SEM). For the ASR experiment, the mean startle amplitude was analyzed by two-way repeated measures analysis of variance (ANOVA) with the factors TIME (pre- and post-treatment) and TREATMENT (OT, OTA or ACSF). For the FPS experiment, the mean startle amplitude was analyzed by two-way repeated measures ANOVA with the factors TRIAL TYPE (pre-shock, noise-alone, and light-noise) and TREATMENT (OT, OTA or ACSF). Where the F-ratio was significant, all-pairwise post hoc comparisons were made using Bonferroni’s tests. The percent change scores (cued fear, and non-cued fear) were analyzed separately for each measure using one-way ANOVA. The effects of drug treatments on shock reactivity during fear conditioning were analyzed by one-way ANOVA. The percent change scores (cued and non-cued fear) and shock reactivity between low and high responders infused with ACSF were calculated with unpaired t-test. The statistical analyses were completed using GraphPad Prism version 6 (GraphPad Software Inc., San Diego, CA). P ≤ 0.05 was considered significant.
3. Results
3.1 Effects of OT or OTA administration into the BNSTdl on the ASR
Quantitative analysis revealed that neither OT nor OTA changed the ASR amplitude when administered intra-BNSTdl 10 min before the test. There was no main effect of TIME (F (1, 18) = 2.92, P = 0.10) or TREATMENT (F (2, 18) = 0.12, P = 0.89), and no interaction between TIME and TREATMENT (F (2, 18) = 0.46, P = 0.64, two-way repeated measures ANOVA) (Fig. 3).
Fig. 3.

Neither OT nor OTA administration into the BNSTdl affects the ASR. Bars show mean (± SEM) startle amplitude before and after bilateral intra-BNSTdl administration of ACSF (0.5 μl in each side, n=7, gray), OT (100 ng in 0.5 μl, n=7, red) or OTA (200 ng in 0.5 μl, n=7, blue).
3.2 Effects of OT or OTA administration into the BNSTdl on the acquisition of FPS
3.2.1 Acquisition of cued fear conditioning
All animals exhibited a significantly potentiated startle response in light-noise trials compared to noise-alone trials. There was a significant main effect of TRIAL TYPE (noise-alone, light-noise) (F (1, 51) = 91.33, P < 0.0001), but no main effect of TREATMENT (F (2, 51) = 0.52, P = 0.60). However, there was a significant interaction between TRIAL TYPE and TREATMENT (F (2, 51) = 5.63, P = 0.0062 two-way repeated measures ANOVA). Post hoc comparisons revealed significant differences in startle amplitude in light-noise trials compared to noise-alone trials in ACSF-treated group (t (20) = 7.78, P < 0.0001), OT-treated group (t (12) = 5.70, P < 0.0001), as well as in OTA-treated rats (t (19) = 3.19, P < 0.01), Bonferroni’s post hoc tests (Fig. 4A, B). The percent change analysis revealed significant effect of TREATMENT on cued fear (F (2, 51) = 4.36, P = 0.017), and Bonferroni’s post hoc comparisons test revealed a significant reduction in cued fear in OTA-treated group compared to ACSF-treated animals (P < 0.05, Fig. 4C).
Fig. 4.

Intra-BNSTdl administration of OTA, but not OT, reduces the acquisition of cued fear. (A) Group data for pre-shock, noise-alone, and light-noise startle amplitude from rats given bilateral intra-BNSTdl ACSF (0.5 μl in each side, n=21, gray), OT (100 ng in 0.5 μl, n=13, red) or OTA (200 ng in 0.5 μl, n=20, blue), 10 min prior to the fear conditioning session. Cued fear: All rats exhibited a significantly potentiated startle response in light-noise trials compared to noise-alone trials - ACSF-treated rats (P < 0.0001), OT-treated (P < 0.0001), and OTA-treated rats (P < 0.01), **** P < 0.0001, ** P < 0.01, Bonferroni’s post hoc tests. Non-cued fear: All rats exhibited a significant potentiation of startle amplitude in noise alone trials in comparison to pre-shock ASR (P < 0.0001), but this was not affected by intra-BNSTdl injections. (B) The mean (± SEM) startle amplitude from ACSF-treated (gray), OT-treated (red), and OTA-treated (blue) rats is shown for grouped 10 noise-alone trials and 10 light-noise trials over the FPS test session. Group data for percent change scores: (C) cued fear shows main effect of TREATMENT (P = 0.017, one-way ANOVA) and a significant reduction of percentage of cued fear in OTA-treated rats in comparison to ACSF-treated rats (Bonferroni’s post hoc tests, * P < 0.05); (D) non-cued fear background anxiety (P = 0.52) for rats given intra-BNSTdl ACSF, OT or OTA. (E) Group data for the shock reactivity amplitude (P = 0.57, one-way ANOVA).
3.2.2 Acquisition of non-cued fear conditioning
Quantitative analysis of the non-cued fear showed a significant enhancement of startle amplitude in noise-alone trials compared to pre-shock trials across all groups. There was a main effect of TRIAL TYPE (F (1, 51) = 20.79, P < 0.0001) but no main effect of TREATMENT (F (2, 51) = 1.39, P = 0.26) and no interaction between TRIAL TYPE and TREATMENT (F (2, 51) = 1.15, P = 0.32) (Fig. 4A). Similarly, the mean percent change in non-cued fear was not different between treatment groups F (2, 51) = 0.66, P = 0.52) (Fig. 4D).
3.2.3 Shock reactivity
The mean shock reactivity during the fear conditioning session was not different between ACSF, OT and OTA-treatment groups (F (2, 51) = 0.57, P = 0.57) (Fig. 4E).
3.3 FPS in rats with low and high levels of pre-shock ASR
Peripherally administered OT was shown to reduce background anxiety measured in FPS only in rats with low levels of ASR (Ayers et al., 2016). To assess whether OT or OTA might differently affect rats with low and high levels of ASR, animals were divided into low and high responders based on their pre-shock ASR values. We used a median split within each experimental group to divide animals into low (ACSF=11, OT=7, OTA=10) and high responders (ACSF=10, OT=6, OTA=10).
In ACSF-treated controls, low and high responders showed equivalent percentage of cued fear and an unpaired t-test comparing the mean percent change in cued fear revealed no significant difference between low and high responders (P = 0.79, Fig. 5A). However, there was a significant difference in percentage of non-cued fear between low and high responders (P = 0.03, unpaired t-test, Fig. 5B). Finally, shock reactivity during fear conditioning session was not significantly different between low and high responders (P = 0.37, unpaired t-test, Fig. 5C).
Fig. 5.

Group data for percent change scores of (A) cued fear (P = 0.79,), (B) non-cued fear (P = 0.03), and (C) shock reactivity amplitude (P = 0.37, unpaired t-test) in rats with low and high levels of pre-shock startle after intra-BNSTdl ACSF injection.
3.3.1 Effects of OT or OTA administration into the BNSTdl on the acquisition of FPS in rats with low levels of pre-shock ASR
In low responders, quantitative analysis showed a significant enhancement of startle amplitude in light-noise trials compared to noise-alone trials such that there was a significant main effect of TRIAL TYPE (noise-alone, light-noise) (F (1, 25) = 68.45, P < 0.0001), but no main effect of TREATMENT (F (2, 25) = 2.27, P = 0.12). However, there was a significant interaction between TRIAL TYPE and TREATMENT (F (2, 25) = 6.87, P = 0.004, two-way repeated measures ANOVA). Post hoc comparisons revealed significant differences in startle amplitude in light-noise trials compared to noise-alone trials in ACSF-treated group (t (10) = 6.01, P < 0.0001), OT-treated group (t (6) = 6.20, P < 0.0001), but not in OTA-treated rats (t (9) = 1.97, P > 0.05), Bonferroni’s test. In addition, the startle amplitude in light-noise trials was significantly different between OT and OTA-treated rats (P < 0.01, Bonferroni’s post hoc tests) (Fig. 6A). Notably, the comparison of mean percent changes in cued fear revealed significant effect of TREATMENT (F (2, 25) = 4.10, P = 0.03) and Bonferroni’s multiple comparisons test showed a significant reduction in cued fear in OTA-treated group in comparison to ACSF-treated group (P < 0.05, Fig. 6B).
Fig. 6.
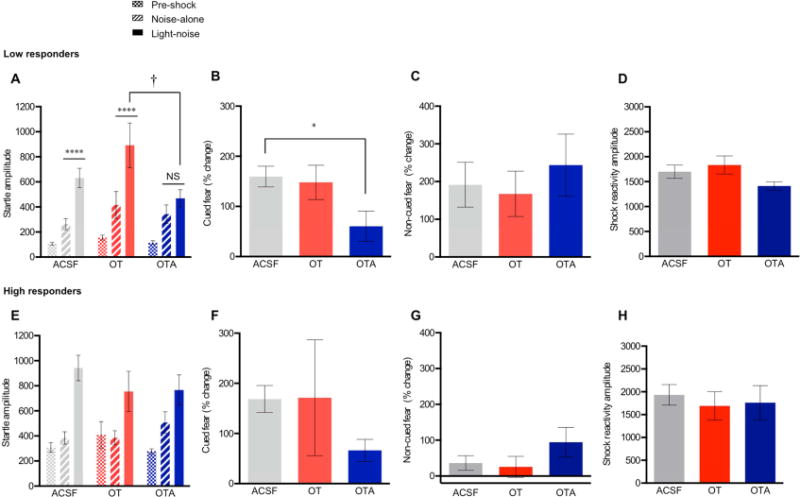
Intra-BNSTdl administration of OTA impairs the acquisition of cued fear in rats with low levels of pre-shock ASR. (A) Group data for pre-shock, noise-alone, and light-noise startle amplitude from low responders given bilateral intra-BNSTdl ACSF (0.5 μl in each side, n=11, gray), OT (100 ng in 0.5 μl, n=7, red) or OTA (200 ng in 0.5 μl, n=10, blue), 10 min prior to the fear conditioning session. Cued fear: In contrast to the ACSF and OT-treated animals (P < 0.0001), OTA-treated rats did not show a significant potentiation of ASR in response to light-noise trials in comparison to noise-alone trials (P > 0.05), **** P < 0.0001 and † P < 0.01, Bonferroni’s post hoc tests. Non-cued fear: All rats show a significant potentiation of ASR in response to noise-alone trials in comparison to pre-shock ASR trials, but this was not affected by treatment. (B) Bars show mean (± SEM) percentage of cued fear in rats given intra-BNSTdl ACSF (gray), OT (red) or OTA (blue). Percentage change of cued fear was significantly affected by TREATMENT (P = 0.03, one-way ANOVA), and Bonferroni’s post hoc test revealed a significant reduction of cued fear in OTA-treated rats in comparison to ACSF-treated rats (* P < 0.05). (C) Bars show mean (± SEM) percentage of non-cued fear in rats given intra-BNSTdl ACSF (gray), OT (red) or OTA (blue) (P = 0.75, ANOVA). (D) Bars show mean (± SEM) shock reactivity during fear-conditioning after intra-BNSTdl ACSF (gray), OT (red) or OTA (blue) injections in low responders (P = 0.097). (E) Group data for pre-shock, noise-alone, and light-noise startle amplitude from high responders given intra-BNSTdl ACSF (n=10), OT (n=6) or OTA (n=10). Cued fear: All high responders demonstrated a significant cued fear, but this was not affected by intra-BNSTdl injections. Non-cued fear: High responders did not develop a significant non-cued fear, and this was not affected by intra-BNSTdl injections. Bars show mean (± SEM) percentage change of cued (F, P = 0.27) and non-cued fear (G, P = 0.30) in rats with high levels of pre-shock startle. (H) Bars show mean (± SEM) shock reactivity during fear-conditioning after intra-BNSTdl ACSF (gray), OT (red) or OTA (blue) injections in high responders (P = 0.86, ANOVA).
Quantitative group analysis revealed a significant potentiation of startle amplitude during noise-alone trials in comparison to pre-shock startle trials (non-cued fear) in all low responders, but this was not affected by intra-BNSTdl injections. There was a significant main effect of TRIAL TYPE (pre-shock ASR, noise-alone) (F (1, 25) = 26.95, P < 0.0001) but no effect of TREATMENT (F (2, 25) = 1.70, P = 0.20), and no interaction between TRIAL TYPE and TREATMENT (F (2, 25) = 0.57, P = 0.57) (Fig. 6A). Comparison of percent changes in background anxiety did not reveal any differences between treatment groups (F (2, 25) = 0.29, P = 0.75, Fig. 6C).
Finally, the shock reactivity during fear conditioning was not affected by intra-BNSTdl injections in rats with low levels of ASR (F (2, 25) = 2.56, P = 0.097, one-way ANOVA, Fig. 6D).
3.3.2 Effects of OT or OTA administration into the BNSTdl on the acquisition of FPS in rats with high levels of pre-shock ASR
In high responders, quantitative analysis revealed a significant cued fear across all groups. There was a significant main effect of TRIAL TYPE (noise-alone, light-noise) (F (1, 23) = 37.14, P < 0.0001) but no main effect of TREATMENT (F (2, 23) = 0.27, P = 0.76), and no interaction between TRIAL TYPE and TREATMENT (F (2, 23) = 2.21, P = 0.13, Fig. 6E). Accordingly, the mean percentage of cued fear was not significantly different among experimental groups (F (2, 23) = 1.38, P = 0.27, one-way ANOVA, Fig. 6F). However, variability observed in a percentage change of cued fear in the OT-treated group of high responders (Fig. 6F) might have resulted in the lack of significant ANOVA. We have therefore recalculated the results after excluding two outliers from the OT-treated group (cued fear: −5.15 % and 737.01 %, respectively). Here, the comparison of mean percent changes in cued fear revealed significant effect of TREATMENT (F (2, 21) = 5.24, P = 0.01) and Bonferroni’s multiple comparisons test showed a significant reduction in cued fear in OTA-treated group in comparison to ACSF-treated group (P < 0.05). Based on the ANOVA results, we cannot exclude the possibility that the effect of OTA on cued fear is observed in both low and high responders.
Quantitative analysis revealed no significant non-cued fear in any group of high responders. There was no main effect of TRIAL TYPE (pre-shock baseline, noise-alone) (F (1, 23) = 2.88, P = 0.10), no main effect of TREATMENT (F (2, 23) = 0.55, P = 0.58), and no interaction between TRIAL TYPE and TREATMENT (F (2, 23) = 1.75, P = 0.20), Fig. 6E). In addition, the comparison of mean percent change in non-cued fear revealed no significant difference between treatment groups (F (2, 23) = 2.26, P = 0.30, one-way ANOVA, Fig. 6G).
Similarly to the low responders, the shock reactivity measured during fear conditioning was not different between treatment groups in high responders (F (2, 23) = 0.15, P = 0.86, one-way ANOVA, Fig. 6H).
3.4 Effects of OT or OTA administration into the BNSTdl on the consolidation of FPS
3.4.1 Consolidation of cued fear conditioning
To determine the role of OT in cued fear consolidation, the rats were injected with ACSF, OT or OTA into the BNSTdl after fear conditioning session. All animals showed increased startle amplitude in light-noise trials compared to noise-alone trials, indicative of robust cued fear. There was a main effect of TRIAL TYPE (noise-alone, light-noise) (F (1, 19) = 24.94, P < 0.0001) but no main effect of TREATMENT (F (2, 19) = 0.17, P = 0.85), and no interaction between TRIAL TYPE and TREATMENT (F (2, 19) = 0.50, P = 0.61, two-way repeated measures ANOVA) (Fig. 7A). The comparison of mean percent change in cued fear revealed no significant difference between treatment groups (F (2, 19) = 1.58, P = 0.23, one-way ANOVA) (Fig. 7B).
Fig. 7.

Neither OT nor OTA administration into the BNSTdl affects the consolidation of FPS. (A) Group data for pre-shock, noise-alone, and light-noise startle amplitude from rats given bilateral intra-BNSTdl ACSF (0.5 μl in each side, n=8, gray), OT (100 ng in 0.5 μl, n=7, red) or OTA (200 ng in 0.5 μl, n=7, blue), 30 min after completing the fear conditioning session. There was a significant increase in startle amplitude in light-noise trials compared to noise-alone trials across all rats (cued fear) but this was not affected by intra-BNSTdl injections. There was no significant increase in startle amplitude in noise-alone trials compared to pre-shock ASR trials across all rats (non-cued fear) and this was not affected by intra-BNSTdl injections. Group data for percent change scores of cued fear (B, P = 0.23) and non-cued fear (C, P = 0.28) for rats given intra-BNST ACSF (gray), OT (red) or OTA (blue).
3.4.2 Consolidation of non-cued fear conditioning
The animals did not show a significant enhancement of startle amplitude in noise-alone trials compared to pre-shock trials. There was no main significant main effect of TRIAL TYPE (pre-shock baseline, noise-alone) (F (1, 19) = 0.30, P = 0.59), and no main effect of TREATMENT (F (2, 19) = 0.39, P = 0.68) or interaction between TRIAL TYPE and TREATMENT (F (2, 19) = 0.006, P = 0.99) (Fig. 7A). The percent change analysis revealed that neither OT nor OTA affected consolidation of non-cued fear (F (2, 19) = 0.42, P = 0.66, one-way ANOVA, Fig. 7C).
4. Discussion
Here, we demonstrate for the first time that intra-BNSTdl administration of OTA, but not OT, prior to the fear conditioning session reduces the acquisition of cued fear in the FPS paradigm. Our results suggest that OTR neurotransmission in the BNSTdl facilitates the acquisition of conditioned fear to a short, discrete cue, without affecting the formation of non-cued fear. The effect of OTA on the acquisition of cued fear was specific to the process of associative learning, because OTA did not affect ability to startle or shock reactivity measured during the fear conditioning session. Furthermore, OTR neurotransmission appears specifically involved in the process of fear acquisition because neither OTA nor OT affected consolidation of the cued fear.
The role of the BNST in mediating conditioned fear responses elicited by long-duration cues (Waddell et al., 2006; Walker et al., 2009b) or context (Duvarci et al., 2009; Sullivan et al., 2004) is well documented. Although initial lesion studies did not associate the BNST with modulation of conditioned fear to short-duration cues (Gewirtz et al., 1998; Hitchcock and Davis, 1991; LeDoux et al., 1988), accumulating evidence suggests otherwise, for review see (Gungor and Pare, 2016). During the recall of classically conditioned fear responses, 25% of BNST neurons show alterations in firing rates in response to a discrete CS (Haufler et al., 2013). Deactivation of the BNST enhances discrimination of CS+ and CS− during conditioned fear responses (Duvarci et al., 2009). Recent studies have shown the critical role of the BNST in serotonin-induced enhancement of cued fear (Marcinkiewcz et al., 2016; Pelrine et al., 2016). These studies suggest that the BNST is also involved in the processing of cued fear conditioning. Notably, the BNST is a heterogeneous structure that contains a variety of neuropeptides (Hazra et al., 2011; Walter et al., 1991), which are critical modulators of behavior, but often exert opposing behavioral effects (Kash et al., 2015). Therefore, specific roles of various inputs and peptidergic neuromodulators in the BNST might not have been revealed by the lesion studies. Our results not only support the role of the BNSTdl in the regulation of fear associated with short-duration cues but also indicate that the OTR transmission in the BNSTdl facilitates the acquisition of cued fear. In the BNSTdl, OTR mRNA is expressed in Type II, electrophysiologically defined neurons (Dabrowska et al., 2011), which are putative inhibitory interneurons (Daniel and Rainnie, 2016). In the CeA, OT was shown to reduce fear expression by activating inhibitory interneurons and enhancing GABA transmission, which results in reduced activity of CeA output neurons (Viviani et al., 2011). It is likely that OTRs in the BNST also regulate fear learning by enhancing GABA transmission, as intra-BNST infusion of GABA-A agonist was shown to potentiate FPS expression (Meloni et al., 2006). Nonetheless, our results suggest that OTR might have opposing roles in modulating fear in the CeA and the BNST, such that it has been shown to reduce fear expression in the CeA (Knobloch et al., 2012) and it appears to facilitate fear acquisition in the BNST. However, OTR transmission might also differentially contribute to distinct phases of fear learning even within the same brain region, like it has been demonstrated in the CeA (Knobloch et al., 2012; Lahoud and Maroun, 2013). As OTRs are also found in Type III, putative CRF neurons in the BNSTdl (Dabrowska et al., 2011), which might be involved in the modulation of fear and anxiety (Dabrowska et al., 2013; Marcinkiewcz et al., 2016); it is possible that OTR on the CRF neurons modulate their neuronal activity and as such regulate the acquisition of cued fear.
In addition to the cued fear, during FPS testing, we have also measured potentiation of the startle amplitude in noise-alone trials that occurs after the first presentation of CS (Walker and Davis, 2002). We referred to this FPS component as non-cued fear but in some FPS studies it has been described as background anxiety (Ayers et al., 2016; Ayers et al., 2011; Missig et al., 2010). This FPS component might represent a slow decay of cued fear from the preceding light-noise trial, which is sustained beyond the immediate threat (Ayers et al., 2011; Missig et al., 2010). More likely, it reflects potentiation of the startle amplitude in response to unpredictable (not signaled by a cue) vs. predictable stimuli (cued-fear) (Grillon et al., 2009). Our results show that OTA selectively impaired potentiation of the startle amplitude during CS+ presentation (cued fear), without affecting startle potentiation during noise-alone trials (CS−, non-cued fear or background anxiety). These results suggest that OTR neurotransmission in the BNSTdl enables learning of the discrimination between light-noise (CS+) and noise-alone (CS−) trials during fear conditioning, as we have shown that blocking OTR during learning process disrupts the ability to differently respond to CS+ and CS− trials during FPS testing. The role of the BNST in mediating such discrimination has been shown before (Duvarci et al., 2009).
More broadly, our results imply that OTRs in the BNSTdl facilitate the formation of adaptive fear response, which allows discrimination between the threatening and non-threatening stimuli. In contrast, OTR blockade does not have an effect on baseline startle response or background anxiety. The latter suggests that OTR in the BNSTdl does not modulate response to an unpredictable threat (non-cued fear), which reflects inability to discriminate between threat and safety (Grillon and Morgan, 1999; Grillon et al., 2009; Rosen and Schulkin, 1998). Relevant to the human FPS studies, the enhanced startle in response to situations of unpredictable threat (aversive stimuli administered unpredictably) is potentiated in patients suffering from post-traumatic stress disorder (PTSD), whereas startle increase in response to predictable threat (signaled by a cue) is not further enhanced in these patients (Grillon et al., 2009).
In the previous FPS studies, systemic, but not ICV, administration of OT reduced background anxiety in rats (Ayers et al., 2011; Missig et al., 2010) without affecting cued or contextual fear. Given that OT does not readily cross the blood brain barrier and OTR are highly expressed in the periphery (Gimpl and Fahrenholz, 2001), systemic OT might trigger peripheral mechanisms that are independent of central OT release (Evans et al., 2014; Moaddab et al., 2015). Therefore, it is difficult to directly compare the effects observed in the current study after intra-BNSTdl infusion to the effects of peripheral OT administration. Although more recent study by the same group did not reproduce the overall effect of OT on background anxiety, it demonstrated differential OT effects on background anxiety in rats with low and high levels of baseline startle (Ayers et al., 2016). Our results show that although background anxiety was not affected by intra-BNSTdl administration of OT or OTA in high or low responders, there was a significant difference in non cued-fear (background anxiety) between low- and high- responders infused with ACSF, such as high responders did not develop a significant potentiation of the startle amplitude in noise-alone trials. Furthermore, although non-cued fear was not affected by any treatment, blockade of OTRs in the BNSTdl impaired the acquisition of cued fear in rats with low, and to a lesser extent, high levels of baseline startle. Importantly, both low and high responders demonstrate equivalent levels of cued fear after ACSF administration. The mechanisms of the potentially different effects of OTA in high and low ASR responders are yet to be determined. However, CRF is one of the major neuromodulators of ASR in the BNST (Lee and Davis, 1997; Walker et al., 2009b), and it might interact with OTR in the BNST (Dabrowska et al., 2011). Hence, it is possible that differences in the baseline CRF transmission between low and high responders might contribute to distinct effects of OTA in the BNSTdl. However, it needs to be noted that high variability of cued fear (percentage change) observed in OT-treated high-responders had inevitably contributed to the lack of significant treatment effect, when analyzed with ANOVA. Based on these results, we cannot unequivocally state that the effect of OTA on cued fear was only observed in low-responding rats.
Previous studies in rodents have shown that background anxiety measure is mainly independent from the contextual fear (Missig et al., 2010). In our design this has been also assured by altering context between FPS training and testing sessions (presence of background noise, removal of shock-conveying grid) to prevent formation of a significant contextual fear. However, future studies are warranted to test the effects of OT and OTA in the BNSTdl on the contextual fear (startle amplitude to noise only measured in the training context without CS presentation). Currently we cannot entirely exclude a possibility that the observed non-cued fear might have been contaminated with contextual fear. In addition, although all rats displayed robust cued fear, non-cued fear (background anxiety) was not significant in the consolidation experiment; hence future studies are warranted to test the potential contribution of OTR neurotransmission to the consolidation of non-cued fear.
OT is currently being tested as a potential pharmacotherapeutic agent for stress-related anxiety disorders, yet the effects of OT on fear learning are often contrasting and dependent on a brain region (Cohen et al., 2010; Guzman et al., 2013; Knobloch et al., 2012; Lahoud and Maroun, 2013) or different phase of fear learning (Toth et al., 2012). Although studies on intranasal OT application for fear-based psychiatric disorders in humans are promising (Acheson et al., 2013; Koch et al., 2016), other studies have shown enhancing effect of OT on fear learning in humans, when given prior to the fear conditioning (acquisition) (Eckstein et al., 2016). Other studies have shown that OT can be anxiogenic in humans as it can potentiate startle reactivity in response to an unpredictable threat (Grillon et al., 2013). Importantly, most of the animal and human studies on the role of OT in the regulation of fear have relied on application of exogenous OT, whereas exposure to various stressors and aversive stimuli triggers central OT release (Ebner et al., 2005; Landgraf and Neumann, 2004). Therefore, in the BNSTdl, foot shock-induced OT release during fear conditioning might have prevented any additional effect of exogenous OT application on fear acquisition in our experiments. Furthermore, our results suggest that rather than in response to a non-specific stressor, like foot shock, OT in the BNSTdl might be released specifically during associative learning during CS-US exposure. In fact, the involvement of OTR neurotransmission specifically during learning process has been vastly reported outside the fear circuitry, especially as it pertains to a social behavior. This includes social recognition mediated by OTR in the posterior BNST (Dumais et al., 2016), pup calls’ recognition mediated by OTR in maternal left auditory cortex (Marlin et al., 2015), and partner preference formation in prairie voles mediated by OTR in the nucleus accumbens (Ross and Young, 2009). Our results indicate that endogenous OT neurotransmission in the BNSTdl is specifically engaged during the formation of cued fear. Here we postulate that as the function of the OT release during fear learning processes remains poorly understood, OTR antagonist studies are needed to better understand the role of endogenous OT system in the regulation of fear vs. anxiety.
In summary, we demonstrate that blocking OTR in the BNSTdl reduces the acquisition, but not consolidation, of conditioned cued fear, yet leaves the baseline startle and non-cued fear (background anxiety) intact. Our study reveals the critical role of OTR neurotransmission in the BNSTdl in the acquisition of conditioned fear to a discrete cue. Future studies will focus on understanding the involvement of distinct neuronal populations in the BNSTdl and specific neurocircuitry mediating the OTR-induced facilitation of cued fear learning.
Highlights.
Oxytocin receptor (OTR) transmission in the BNST facilitates acquisition of cued fear
OTRs in the BNST do not contribute to consolidation of cued fear
Manipulation of OTR in the BNST does not affect baseline startle or non-cued fear
Acknowledgments
We thank Dr. David L. Walker, PhD, for helpful suggestions and discussions. We also thank Mrs. Daisy Martinon, MSc, for excellent technical assistance.
Funding and disclosures
This work was supported by Grant R00 MH-096746 and R01 MH113007 from the National Institute of Mental Health to JD and start-up funds from the Chicago Medical School, Rosalind Franklin University of Medicine Science to JD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson D, Feifel D, de Wilde S, McKinney R, Lohr J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology (Berl) 2013;229:199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers L, Agostini A, Schulkin J, Rosen JB. Effects of oxytocin on background anxiety in rats with high or low baseline startle. Psychopharmacology (Berl) 2016;233:2165–2172. doi: 10.1007/s00213-016-4267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: peripheral vs central administration. Neuropsychopharmacology. 2011;36:2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010;22:889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol Psychiatry. 2013;74:817–826. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG. Targeting corticotropin-releasing factor (CRF) projections from the oval nucleus of the BNST using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol. 2016 doi: 10.1111/jne.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology. 2016;41:103–125. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL. Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct. 2014;219:1969–1982. doi: 10.1007/s00429-013-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Pare D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Patin A, Preckel K, Becker B, Walter A, Domschke K, Grinevich V, Maier W, Hurlemann R. Oxytocin Facilitates Pavlovian Fear Learning in Males. Neuropsychopharmacology. 2016;41:932–939. doi: 10.1038/npp.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Cardoso C, Joober R. Intranasal oxytocin attenuates the human acoustic startle response independent of emotional modulation. Psychophysiology. 2014;51:1169–1177. doi: 10.1111/psyp.12263. [DOI] [PubMed] [Google Scholar]
- Evans SL, Dal Monte O, Noble P, Averbeck BB. Intranasal oxytocin effects on social cognition: a critique. Brain Res. 2014;1580:69–77. doi: 10.1016/j.brainres.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18:958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Pare D. Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J Neurosci. 2016;36:8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Ryan SJ, Jasnow AM, Dabrowska J, Rainnie DG. A transcriptomic analysis of type I–III neurons in the bed nucleus of the stria terminalis. Mol Cell Neurosci. 2011;46:699–709. doi: 10.1016/j.mcn.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells. 2015;38:1–13. doi: 10.14348/molcells.2015.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:2041–2051. doi: 10.1038/npp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud N, Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology. 2013;38:2184–2195. doi: 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA., Jr Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Ann N Y Acad Sci. 2006;1071:538–541. doi: 10.1196/annals.1364.059. [DOI] [PubMed] [Google Scholar]
- Missig G, Ayers LW, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacology. 2010;35:2607–2616. doi: 10.1038/npp.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddab M, Hyland BI, Brown CH. Oxytocin enhances the expression of morphine-induced conditioned place preference in rats. Psychoneuroendocrinology. 2015;53:159–169. doi: 10.1016/j.psyneuen.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol Psychiatry. 2016;79:213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: 2007. [DOI] [PubMed] [Google Scholar]
- Pelrine E, Pasik SD, Bayat L, Goldschmiedt D, Bauer EP. 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors. Neurobiol Learn Mem. 2016;136:189–195. doi: 10.1016/j.nlm.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Jennings JH, Ung RL, Stuber GD. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav Brain Res. 2013;255:19–25. doi: 10.1016/j.bbr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, Slattery DA. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology (Berl) 2012;223:149–158. doi: 10.1007/s00213-012-2702-4. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009a;34:1533–1542. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009b;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Mai JK, Lanta L, Gorcs T. Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chem Neuroanat. 1991;4:281–298. doi: 10.1016/0891-0618(91)90019-9. [DOI] [PubMed] [Google Scholar]


