ABSTRACT
Recently reported shelf-stable, on-demand protein synthesis platforms are enabling new possibilities in biotherapeutics, biosensing, biocatalysis, and high throughput protein expression. Lyophilized cell-free protein expression systems not only overcome cold-storage limitations, but also enable stockpiling for on-demand synthesis and completely sterilize the protein synthesis platform. Recently reported high-yield synthesis of cytotoxic protein Onconase from lyophilized E. coli extract preparations demonstrates the utility of lyophilized cell-free protein expression and its potential for creating on-demand biotherapeutics, vaccines, biosensors, biocatalysts, and high throughput protein synthesis.
KEYWORDS: cell-free, cfps, freeze dry, lyophilize, onconase, protein expression, protein synthesis, tx-tl
Introduction
Custom protein synthesis is a 50+ billion USD industry that impacts most parts of human lives including the clothes we wear, the food we eat, the beverages we drink, and the lifesaving therapeutics many require.1 The original workhorse of custom recombinant protein synthesis is Escherichia coli, which remains the most cost-effective method and can produce many complex proteins (including insulin2 and antibodies3). Other synthesis chassis including yeast and mammalian cells have been developed for proteins requiring advanced assembly and post-translational modifications such as glycosylation and lipidation.4,5
Additionally, cell-free protein expression systems have introduced many attractive advantages to protein synthesis technology.6 Because the reaction environment is not confined within a cell wall, proteins can be expressed from rapidly-produced PCR gene products,7 and dynamic optimization of the reaction environment becomes possible.8,9 Changes in reaction conditions such as redox potential, pH, hydrophobicity, and temperature enable expression of a variety of active proteins that are often difficult to produce in vivo. Innovative examples include cytotoxic proteins,10 membrane proteins,11,12 metallic holoenzymes,13,14 and virus-like particles.15-17 Cell-free protein synthesis (CFPS) reactions also allow the incorporation of unnatural, even toxic18 amino acids.19-23
A major limitation of both in vivo and cell-free protein expression methods is the cold storage chain essential for chassis storage. Lyophilizing, or freeze drying, is a common technique for rendering biochemical and bioactive mixtures stable. Lyophilization of wheat-germ extracts and its activity in cell-free protein expression has been reported.24 Inspired by this report and seeking to overcome scale-up and RNA stabilization challenges of this system, we created a lyophilized cell-free protein synthesis system based on E. coli cell extracts for shelf-stable storage and transportation.25 The system, in addition to breaking the cold-storage chain, has the added benefits of 1) a just-add-water + DNA format where protein can be produced rapidly on-demand – in as little as 1 h, 2) consistent scalable production from 250 µL up to 100 L26, and 3) sterilization of the system to prevent release of residual genetically modified bacteria if transported or used in-field.27
Thus shelf-stable, on-demand protein synthesis platforms are enabling new possibilities in biotechnology applications. As a particularly innovative example, Pardee et al. have applied lyophilized cell-free systems to enable rapid detection of Ebola and Zika viruses. This cell-free platform was lyophilized into a paper support, and when reconstituted, allowed portable virus detection.28,29 We recently reported the on-demand production of the cytotoxic cancer therapeutic Onconase from a lyophilized cell-free protein expression system, which allows for shelf-stable storage of the extract for 90 d before use. The resultant protein was produced at high yields and was immediately available for screening without purification.10 In this article, we will highlight technological advances and future applications enabled by lyophilized, on-demand protein expression systems.
Therapeutic production from shelf-stable expression system: Onconase10
Onconase (ranpirnase) is a potent biotherapeutic that has been used to treat cancer and viral infections.30,31 Its cytotoxic mechanism of action is the degradation of tRNA, which paralyzes protein production.4 As a result, high-yield production in living cells is only possible if the protein folds incorrectly into inclusion bodies directly following synthesis. Refolding requires several steps and multiple days to recover some of the original activity.32,33
Initial expression of Onconase in E. coli cell-free protein synthesis (CFPS) reactions resulted in 80% of produced Onconase being soluble, likely attributed to CFPS's slower expression rates and 25-fold less-crowded environment.34 However, initial protein yields were less than 3% of those obtained with model protein GFP through E. coli CFPS (0.03 mg/mL of Onconase compared to 1.45 mg/mL of model protein GFP). To address the hypothesized tRNA degradation caused by Onconase, tRNA was systematically added to the open CFPS reaction environment. This resulted in an overall Onconase yield of 1.86 mg/mL with more than 95% being soluble. It is also significant to note that the estimated production cost of Onconase is less than 30 USD per milligram.
Following cell-free synthesis, Onconase was added without purification to a breast cancer cell line (MCF-7) and assayed for cell viability. Controls also added to MCF-7 included refolded Onconase expressed in E. coli, doxorubicin, and cell-free reagents without Onconase. Cell-free produced Onconase inhibited cancer cell growth 60 times more effectively than refolded Onconase and slightly more effectively than doxorubicin.
In order to demonstrate the enhanced flexibility afforded by lyophilization, Onconase was then synthesized from lyophilized extracts. Onconase yields from lyophilized extracts compared favorably with those from standard extracts, matching or exceeding the standard extract Onconase yields. Taken with our previous results, which showed extract viability after up to 90 days storage at room temperature,25 these results demonstrate that a difficult-to-express therapeutic could be produced on-demand, even in remote locations using lyophilized extracts stored at sub-optimal conditions.
Future applications of shelf-stable expression systems
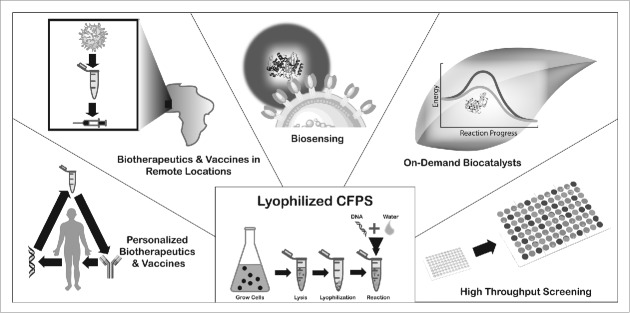
The ability to render protein expression systems shelf-stable enables many exciting applications for cell-free protein synthesis. Among these are personalized and on-demand biotherapeutics and vaccines, biotherapeutics and vaccine production in remote locations, biosensing, on-demand biocatalysis in chemical supply chains, and high throughput protein production for screening, engineering, and protein evolution (Fig. 1).
Figure 1.
Future applications of shelf-stable protein expression systems. Lyophilized protein expression reactions are activated by the addition of water and nucleic acid, and have the potential to enable innovative applications in personalized biotherapeutics and vaccines, biotherapeutic and vaccine production in remote locations, biosensing, on-demand biocatalysis, and high throughput production.
Personalized biotherapeutics and vaccines
The process of creating personalized vaccines typically involves isolating nucleic acid from the patient's affected cells in order to express proteins that are then administered to the patient. These proteins take the form of antigens designed to elicit an immune response. Researchers have already demonstrated the creation of personalized therapeutic vaccines for the treatment of lymphoma using cell-free protein synthesis systems.35,36 Especially in cases of late-stage cancer diagnosis where the time window for effective treatment is severely restricted, rapid and on-demand protein generation is critical. Cancer vaccines have the potential to become increasingly effective and available with the ability to stockpile lyophilized CFPS systems. In addition to creating cancer vaccines, stockpiling of protein expression systems enables rapid vaccine production in response to a viral pandemic threat.37 Shelf-stable protein expression may additionally impact dendritic cell immunotherapy techniques,38 enable effective immunotherapy for combating warts,39 and supply rapid purification-free production of vectors for gene delivery.40,41
Biotherapeutics and vaccines in remote locations
As modern medical treatments depend increasingly on biotherapeutics, the ability to synthesize proteins in remote and adverse environments will be invaluable. Shelf-stable protein expression systems allow for economic transportation and enable the utilization of biotherapeutics in remote locations. Travelers, humanitarians, and defense units could be outfitted with pre-assembled, just-add-water kits similar to the systems used to produce Onconase. Additionally, lyophilized systems make vaccine production technologies available for generation in remote locations, e.g. in response to an epidemic or in other humanitarian efforts.
Biosensing
Biosensors are biochemical constructs designed to indicate the presence of a given analyte, and utilize the specificity of a bioreceptor. Biosensor designs that require active proteins in their operations are beginning to be impacted by portable and on-demand CFPS systems. For example, Pardee et al28 utilized lyophilization to integrate cell-free protein expression triggered by riboregulators into a paper-based biosensor platform. A chromogenic indicator, visible to the naked eye, was engineered to indicate a positive response from the biosensor. The result was a portable assay, activated simply by adding water and a sample of specific RNA sequence. Applying this previously engineered technology, the authors created a highly specific biosensor for Ebola and Zika. The entire creation process, from in silico design to in vitro naked-eye detection, required less than 6 d. It is also significant to note that the diagnostic test itself, including sample collection, RNA extraction, and cell-free reaction requires about 3 h to execute, and the lyophilized biosensor platform is shelf-stable for up to one year.
On-demand biocatalysts
Lyophilized CFPS reactions can also be used to create on-demand biocatalysts, fortifying chemical supply pipelines with increased versatility and economy. Biocatalysts are leading the effort for “green chemistry” in the chemicals industry, eliminating harsh chemical catalysts and waste streams while reducing thermal energy costs. With biocatalysts that can be rapidly synthesized, chemical supply pipelines can be altered in response to a change in the demand of a chemical commodity. Thus chemical feedstock can potentially be more efficiently used to create the chemical product that is most profitable.
High throughput production
Increasingly rapid gene synthesis technology combined with shelf-stable on-demand protein synthesis is a powerful tool for high throughput protein production. Currently, gene product structure and function understanding lags far behind available gene sequence data. Methods for high throughput and economic screening of protein gene products are a significant advantage to researchers seeking to decipher the proteome.42,43 High throughput protein synthesis is also indispensable in rational protein engineering and directed protein evolution. Because standard cell-free extracts must be used quickly or stored frozen, and because preparation of the extracts requires several hours, dependency on such has the potential to bottle-neck execution of rapid protein expression cycles. On-demand protein expression systems, that transcribe and translate from rapidly-produced DNA44 and facilitate activity assessment without product purification, enable parallel synthesis cycles to be executed in as little as 3 to 4 h.10
Conclusion
In an emerging epoch of novel protein systems engineered to meet human needs, technologies providing on-demand protein expression will become fundamentally useful. From cytotoxic cancer therapeutics to Zika virus detectors and on-demand biocatalysts, lyophilized cell-free protein synthesis platforms place exciting new tools in the hands of biotechnologists.
Abbreviations
- CFPS
cell-free protein synthesis
- GFP
green fluorescent protein
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We would like to acknowledge and thank our funding sources for their generous contribution including the National Science Foundation CBET Division CAREER Award (#1254148) and the National Science Foundation Graduate Research Fellowship Program (Grant #1247046). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
References
- [1].Kirk O, Borchert TV, Fuglsang CC. Industrial enzyme applications. Curr Opin Biotechnol 2002; 13:345-51; PMID:12323357; https://doi.org/ 10.1016/S0958-1669(02)00328-2 [DOI] [PubMed] [Google Scholar]
- [2].Baeshen NA, Baeshen MN, Sheikh A, Bora RS, Ahmed MM, Ramadan HA, Saini KS, Redwan EM, Saini KS, Redwan EM. Cell factories for insulin production. Microb Cell fact 2014; 13:141; PMID:25270715; https://doi.org/ 10.1186/s12934-014-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang CJ, Lin H, Yang X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J Ind Microbiol Biotechnol 2012; 39:383-99; PMID:22252444; https://doi.org/ 10.1007/s10295-011-1082-9 [DOI] [PubMed] [Google Scholar]
- [4].Zhao HL, He Q, Xue C, Sun B, Yao XQ, Liu ZM. Secretory expression of glycosylated and aglycosylated mutein of onconase from Pichia pastoris using different secretion signals and their purification and characterization. FEMS Yeast Res 2009; 9:591-9; PMID:19416372; https://doi.org/ 10.1111/j.1567-1364.2009.00498.x [DOI] [PubMed] [Google Scholar]
- [5].Kim JY, Kim YG, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol and Biotechnol 2012; 93:917-30; https://doi.org/ 10.1007/s00253-011-3758-5 [DOI] [PubMed] [Google Scholar]
- [6].Smith MT, Wilding KM, Hunt JM, Bennett AM, Bundy BC. The emerging age of cell-free synthetic biology. FEBS Lett 2014; 588:2755-61; PMID:24931378; https://doi.org/ 10.1016/j.febslet.2014.05.062 [DOI] [PubMed] [Google Scholar]
- [7].Schinn SM, Broadbent A, Bradley WT, Bundy BC. Protein synthesis directly from PCR: progress and applications of cell-free protein synthesis with linear DNA. N Biotechnol 2016; 33:480-7; PMID:27085957; https://doi.org/ 10.1016/j.nbt.2016.04.002 [DOI] [PubMed] [Google Scholar]
- [8].Shrestha P, Holland TM, Bundy BC. Streamlined extract preparation for Escherichia coli-based cell-free protein synthesis by sonication or bead vortex mixing. Biotechniques 2012; 53:163-74; PMID:22963478 [DOI] [PubMed] [Google Scholar]
- [9].Salehi ASM, Earl CC, Muhlestein C, Bundy BC. Escherichia coli-based cell-free extract development for protein-based cancer therapeutic production. Int J Dev Biol 2016; In Press; https://doi.org/ 10.1387/ijdb.160125bb [DOI] [PubMed] [Google Scholar]
- [10].Salehi ASM, Smith MT, Bennett AM, Williams JB, Pitt WG, Bundy BC. Cell-free protein synthesis of a cytotoxic cancer therapeutic: Onconase production and a just-add-water cell-free system. Biotechnol J 2016; 11:274-81; PMID:26380966; https://doi.org/ 10.1002/biot.201500237 [DOI] [PubMed] [Google Scholar]
- [11].Junge F, Haberstock S, Roos C, Stefer S, Proverbio D, Dotsch V, Bernhard F. Advances in cell-free protein synthesis for the functional and structural analysis of membrane proteins. N Biotechnol 2011; 28:262-71; PMID:20637904; https://doi.org/ 10.1016/j.nbt.2010.07.002 [DOI] [PubMed] [Google Scholar]
- [12].Wuu JJ, Swartz JR. High yield cell-free production of integral membrane proteins without refolding or detergents. Biochim Biophys Acta 2008; 1778:1237-50; PMID:18295592; https://doi.org/ 10.1016/j.bbamem.2008.01.023 [DOI] [PubMed] [Google Scholar]
- [13].Li J, Lawton TJ, Kostecki JS, Nisthal A, Fang J, Mayo SL, Rosenzweig AC, Jewett MC. Cell-free protein synthesis enables high yielding synthesis of an active multicopper oxidase. Biotechnol J 2016; 11:212-8; PMID:26356243; https://doi.org/ 10.1002/biot.201500030 [DOI] [PubMed] [Google Scholar]
- [14].Kuchenreuther JM, Shiigi SA, Swartz JR. Cell-free synthesis of the H-cluster: a model for the in vitro assembly of metalloprotein metal centers. Methods Mol Biol 2014; 1122:49-72; PMID:24639253; https://doi.org/ 10.1007/978-1-62703-794-5_5 [DOI] [PubMed] [Google Scholar]
- [15].Smith MT, Varner CT, Bush DB, Bundy BC. The incorporation of the A2 protein to produce novel Qβ virus-like particles using cell-free protein synthesis. Biotechnol Prog 2012; 28:549-55; PMID:22125293; https://doi.org/ 10.1002/btpr.744 [DOI] [PubMed] [Google Scholar]
- [16].Smith MT, Hawes AK, Bundy BC. Reengineering viruses and virus-like particles through chemical functionalization strategies. Curr Opin Biotechnol 2013; 24:620-6; PMID:23465756; https://doi.org/ 10.1016/j.copbio.2013.01.011 [DOI] [PubMed] [Google Scholar]
- [17].Bundy BC, Swartz JR. Efficient disulfide bond formation in virus-like particles. J Biotechnol 2011; 154:230-9; PMID:21536082; https://doi.org/ 10.1016/j.jbiotec.2011.04.011 [DOI] [PubMed] [Google Scholar]
- [18].Worst EG, Exner MP, De Simone A, Schenkelberger M, Noireaux V, Budisa N, Ott A. Cell-free expression with the toxic amino acid canavanine. Bioorg Med Chem Lett 2015; 25:3658-60; PMID:26130409; https://doi.org/ 10.1016/j.bmcl.2015.06.045 [DOI] [PubMed] [Google Scholar]
- [19].Smith MT, Wu JC, Varner CT, Bundy BC. Enhanced protein stability through minimally invasive, direct, covalent, and site-specific immobilization. Biotechnol Prog 2013; 29:247-54; PMID:23225632; https://doi.org/ 10.1002/btpr.1671 [DOI] [PubMed] [Google Scholar]
- [20].Quast RB, Mrusek D, Hoffmeister C, Sonnabend A, Kubick S. Cotranslational incorporation of non-standard amino acids using cell-free protein synthesis. FEBS Lett 2015; 589:1703-12; PMID:25937125; https://doi.org/ 10.1016/j.febslet.2015.04.041 [DOI] [PubMed] [Google Scholar]
- [21].Wu JCY, Hutchings CH, Lindsay MJ, Werner CJ, Bundy BC. Enhanced enzyme stability through site-directed covalent immobilization. J Biotechnol 2015; 193:83-90; PMID:25449015; https://doi.org/ 10.1016/j.jbiotec.2014.10.039 [DOI] [PubMed] [Google Scholar]
- [22].Shrestha P, Smith MT, Bundy BC. Cell-free unnatural amino acid incorporation with alternative energy systems and linear expression templates. N Biotechnol 2014; 31:28-34; PMID:24103470; https://doi.org/ 10.1016/j.nbt.2013.09.002 [DOI] [PubMed] [Google Scholar]
- [23].Smith MT, Hawes AK, Shrestha P, Rainsdon JM, Wu JC, Bundy BC. Alternative fermentation conditions for improved Escherichia coli-based cell-free protein synthesis for proteins requiring supplemental components for proper synthesis. Process Biochem 2014; 49:217-22; https://doi.org/ 10.1016/j.procbio.2013.10.012 [DOI] [Google Scholar]
- [24].Madono M, Sawasaki T, Morishita R, Endo Y. Wheat germ cell-free protein production system for post-genomic research. N Biotechnol 2011; 28:211-7; PMID:20800705; https://doi.org/ 10.1016/j.nbt.2010.08.009 [DOI] [PubMed] [Google Scholar]
- [25].Smith MT, Berkheimer SD, Werner CJ, Bundy BC. Lyophilized Escherichia coli-based cell-free systems for robust, high-density, long-term storage. Biotechniques 2014; 56:186-93; PMID:24724844; https://doi.org/ 10.2144/000114158 [DOI] [PubMed] [Google Scholar]
- [26].Zawada JF, Yin G, Steiner AR, Yang J, Naresh A, Roy SM, Gold DS, Heinsohn HG, Murray CJ. Microscale to manufacturing scale-up of cell-free cytokine production–a new approach for shortening protein production development timelines. Biotechnol Bioeng 2011; 108:1570-8; PMID:21337337; https://doi.org/ 10.1002/bit.23103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Smith MT, Bennett AM, Hunt JM, Bundy BC. Creating a Completely “Cell-free” System for Protein Synthesis. Biotechnol Prog 2015; 31:1716-9; PMID:26289032; https://doi.org/ 10.1002/btpr.2157 [DOI] [PubMed] [Google Scholar]
- [28].Pardee K, Green AA, Ferrante T, Cameron DE, DaleyKeyser A, Yin P, Collins JJ. Paper-based synthetic gene networks. Cell 2014; 159:940-54; PMID:25417167; https://doi.org/ 10.1016/j.cell.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M, et al.. Rapid, low-cost detection of zika virus using programmable biomolecular components. Cell 2016; 165:1255-66; PMID:27160350; https://doi.org/ 10.1016/j.cell.2016.04.059 [DOI] [PubMed] [Google Scholar]
- [30].Costanzi J, Sidransky D, Navon A, Goldsweig H. Ribonucleases as a novel pro-apoptotic anticancer strategy: review of the preclinical and clinical data for ranpirnase. Cancer Invest 2005; 23:643-50; PMID:16305992; https://doi.org/ 10.1080/07357900500283143 [DOI] [PubMed] [Google Scholar]
- [31].Youle RJ, Wu YN, Mikulski SM, Shogen K, Hamilton RS, Newton D, D'Alessio G, Gravell M. RNase inhibition of human immunodeficiency virus infection of H9 cells. Proc Natl Acad Sci U S A 1994; 91:6012-6; PMID:8016107; https://doi.org/ 10.1073/pnas.91.13.6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Notomista E, Cafaro V, Fusiello R, Bracale A, D'Alessio G, Di Donato A. Effective expression and purification of recombinant onconase, an antitumor protein. FEBS Lett 1999; 463:211-5; PMID:10606723; https://doi.org/ 10.1016/S0014-5793(99)01623-3 [DOI] [PubMed] [Google Scholar]
- [33].Pane K, Durante L, Pizzo E, Varcamonti M, Zanfardino A, Sgambati V, Di Maro A, Carpentieri A, Izzo V, Di Donato A, et al.. Rational design of a carrier protein for the production of recombinant toxic peptides in escherichia coli. Plos One 2016; 11:23; https://doi.org/ 10.1371/journal.pone.0146552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Underwood KA, Swartz JR, Puglisi JD. Quantitative polysome analysis identifies limitations in bacterial cell-free protein synthesis. Biotechnol Bioeng 2005; 91:425-35; PMID:15991235; https://doi.org/ 10.1002/bit.20529 [DOI] [PubMed] [Google Scholar]
- [35].Yang J, Kanter G, Voloshin A, Michel-Reydellet N, Velkeen H, Levy R, Swartz JR. Rapid expression of vaccine proteins for B-cell lymphoma in a cell-free system. Biotechnol Bioeng 2005; 89:503-11; PMID:15669088; https://doi.org/ 10.1002/bit.20283 [DOI] [PubMed] [Google Scholar]
- [36].Ng PP, Jia M, Patel KG, Brody JD, Swartz JR, Levy S, Levy R. A vaccine directed to B cells and produced by cell-free protein synthesis generates potent antilymphoma immunity. Proc Natl Acad Sci U S A 2012; 109:14526-31; PMID:22875703; https://doi.org/ 10.1073/pnas.1211018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci U S A 2014; 111:125-30; PMID:24344259; https://doi.org/ 10.1073/pnas.1308701110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bryan JT, Buckland B, Hammond J, Jansen KU. Prevention of cervical cancer: journey to develop the first human papillomavirus virus-like particle vaccine and the next generation vaccine. Curr Opin Chem Biol 2016; 32:34-47; PMID:26994695; https://doi.org/ 10.1016/j.cbpa.2016.03.001 [DOI] [PubMed] [Google Scholar]
- [39].Sharma R, Sharma CL. Quadrivalent human papillomavirus recombinant vaccine: the first vaccine for cervical cancers. J Cancer Res Ther 2007; 3:92-5; PMID:17998730; https://doi.org/ 10.4103/0973-1482.34686 [DOI] [PubMed] [Google Scholar]
- [40].Kanagavelu S, Termini JM, Gupta S, Raffa FN, Fuller KA, Rivas Y, Philip S, Kornbluth RS, Stone GW. HIV-1 adenoviral vector vaccines expressing multi-trimeric BAFF and 4-1BBL enhance T cell mediated anti-viral immunity. PLoS One 2014; 9:e90100; PMID:24587225; https://doi.org/ 10.1371/journal.pone.0090100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu Y, Chan W, Ko BY, VanLang CC, Swartz JR. Assessing sequence plasticity of a virus-like nanoparticle by evolution toward a versatile scaffold for vaccines and drug delivery. Proc Natl Acad Sci U S A 2015; 112:12360-5; PMID:26392546; https://doi.org/ 10.1073/pnas.1510533112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Murray CJ, Baliga R. Cell-free translation of peptides and proteins: from high throughput screening to clinical production. Curr Opin Chem Biol 2013; 17:420-6; PMID:23499386; https://doi.org/ 10.1016/j.cbpa.2013.02.014 [DOI] [PubMed] [Google Scholar]
- [43].Takahashi MK, Hayes CA, Chappell J, Sun ZZ, Murray RM, Noireaux V, et al.. Characterizing and prototyping genetic networks with cell-free transcription-translation reactions. Methods 2015; 86:60-72; PMID:26022922; https://doi.org/ 10.1016/j.ymeth.2015.05.020 [DOI] [PubMed] [Google Scholar]
- [44].Woodrow KA, Airen IO, Swartz JR. Rapid expression of functional genomic libraries. J Proteome Res 2006; 5:3288-300; PMID:17137330; https://doi.org/ 10.1021/pr050459y [DOI] [PubMed] [Google Scholar]