Abstract
Two strategies leading to enzyme saving during saccharification of pretreated lignocellulo-starch biomass (LCSB) was investigated which included reducing enzyme dosage by varying their levels in enzyme cocktails and enhancing the fermentable sugar yield in enzyme-reduced systems using detoxification chemicals. Time course release of reducing sugars (RS) during 24–120 h was significantly higher when an enzyme cocktail containing full dose of cellulase (16 FPU/g cellulose) along with half dose each of xylanase (1.5 mg protein/g hemicelluloses) and Stargen (12.5 μl/g biomass) was used to saccharify conventional dilute sulphuric acid (DSA) pretreated biomass compared to a parallel system where only one-fourth the dose of the latter two enzymes was used. The reduction in RS content in the 120 h saccharified mash to the extent of 3–4 g/L compared to the system saccharified with full complement of the three enzymes could be overcome considerably by supplementing the system (half dose of two enzymes) with detoxification chemical mix incorporating Tween 20, PEG 4000 and sodium borohydride. Microwave (MW)-assisted DSA pretreated biomass on saccharification with enzyme cocktail having full dose of cellulase and half dose of Stargen along with detoxification chemicals gave significantly higher RS yield than DSA pretreated system saccharified using three enzymes. The study showed that xylanase could be eliminated during saccharification of MW-assisted DSA pretreated biomass without affecting RS yield when detoxification chemicals were also supplemented. The Saccharification Efficiency and Overall Conversion Efficiency were also high for the MW-assisted DSA pretreated biomass. Since whole slurry saccharifcation of pretreated biomass is essential to conserve fermentable sugars in LCSB saccharification, detoxification of soluble inhibitors is equally important as channelling out of insoluble lignin remaining in the residue. As one of the major factors contributing to the cost of ethanol production from LCSB is the cost of enzymes, appropriate modification of enzyme cocktail based on the composition of the pretreated biomass coupled with effective detoxification of the slurry would be a promising approach towards cost reduction.
Keywords: Energy, Environmental science, Agriculture, Biotechnology, Biochemistry
1. Introduction
Petroleum-based economy of nations is continuously challenged by the rapidly squeezing natural resources such as oil, natural gas and coal coupled with the climate change related issues affecting human life and agriculture (Sarkar et al., 2012; Zhang and Shahbazi, 2011). The most promising alternative is to switch over to bioethanol as a fuel source due to its several advantages such as high-octane number avoiding the need for the toxic methyl tertiary butyl ether in petrol, high oxygen content reducing the emission of carbon monoxide and non-combusted hydrocarbons, ease of blending with petrol etc. (Farrel et al., 2006; Öhgren et al., 2006). Although the production of bioethanol from sugar or starch containing feedstock is advantageous because of the ease of processing, the shortage of food resources has led to the food-fuel conflict necessitating the lookout for alternative substrates. Lignocellulosic biomass has been documented as the best raw material for production of second generation (2G) ethanol due to the cheap and abundant availability. Nevertheless, the sustainable production depends on how effectively the technological barriers such as biomass recalcitrance, rigorous pretreatment methods shifting the overall process economy to negative side, enzyme costs etc. are overcome (Himmel et al., 2007; Wyman, 1999). Extensive studies have been taken up in the past on biomass residues of global importance such as corn stover, sugarcane bagasse or wheat straw, while other agricultural residues such as rice straw, barley straw, dedicated grasses viz., switchgrass, Miscanthus sp., Bermuda grass etc. also have received research focus (Benjamin et al., 2014; Bussamra et al., 2015; Chen and Liu, 2007; Martin et al., 2002; Mosier et al., 2005; Saha and Cotta, 2009). Although there are some studies on the utilization of municipal waste and other biodegradable household wastes for bioethanol production (Li et al., 2007; Lissens et al., 2004), potential of several agricultural residues still remains to be explored. Vegetable wastes have been reported as ideal feedstock for ethanol production due to their high starch content, besides cellulose and hemicelluloses and also because of their non-competitiveness with food sources (Tang et al., 2008). Non-judicious disposal of such wastes in public areas especially in less developed countries is a matter of concern due to the environmental threats from microbial decay leading to foul odour and harbouring of pests such as rats, pigs or birds making the population vulnerable to various communicable diseases (Singh et al., 2012). Previous studies explored the compositional variations of processing residues such as peels of various root crops (sweet potato, elephant foot yam, tannia, greater yam or beetroot) and vegetables (pumpkin and vegetable banana), pretreatment related changes in composition and ultrastructure as well as optimization of enzyme (cellulose) dosage during saccharification of pretreated residues using response surface methodology (Mithra and Padmaja, 2016a; 2017; Mithra et al., 2017a). These wastes also contain appreciable quantities of starch (25–37%) necessitating different pretreatment and saccharification approaches for them compared to the typical lignocellulosic biomass (LCB). Although pretreatment is obligatory to overcome biomass recalcitrance, the long pretreatment periods and high temperature associated with dilute sulphuric acid (DSA) pretreatment could lead to loss of fermentable sugars by conversion to furfural or 5-hydroxymethyl furfural (Palmqvist and Hahn-Hägerdal, 2000). Lignin degradation products (phenolic compounds) are also reported to be inhibitory to both cellulases and fermentation organisms (Larsson et al., 2000; Tejirian and Xu, 2011; Ximenes et al., 2010). Mild pretreatment conditions such as microwave-assisted acid/alkali pretreatment have been attempted by several researchers to overcome such barriers (Ethaib et al., 2015; Kuhnel et al., 2011; Li et al., 2016; Zhu et al., 2012), although mild conditions result in incomplete hydrolysis of hemicelluloses (xylan) forming xylo-oligomers in the pretreated liquor which are reported as more inhibitory to cellulases than furan derivatives or phenolic compounds (Qing et al., 2010). Earlier studies on peels of root and vegetables (lignocellulo-starch biomass, LCSB) also showed that the optimum conditions for MW-assisted DSA pretreatment were 600 W irradiation power, 7 min processing time and 0.1 mol/L H2SO4 acid concentration. Very high conversion efficiency (85–96%) of carbohydrates to reducing sugars could be achieved under these conditions using a triple enzyme cocktail for saccharification (Mithra et al., 2017b).
Although enzymatic hydrolysis is considered as the most effective saccharification approach yielding high fermentable sugars, it is also critical in the cost-effective production of ethanol (Wyman, 1999; Sun and Cheng, 2002). There are several factors affecting the saccharification efficiency of enzymes such as feedback inhibition by end products, inhibition by toxic components generated during pretreatment, mechanism and interaction of enzymes etc. (Mansfield et al., 1999). Furthermore, the non-productive binding of lignin to cellulases has been reported to significantly reduce the saccharification efficiency, necessitating the addition of more quantity of enzymes which ultimately enhances the cost (Eriksson et al., 2002; Kurakake et al., 1994). Presence of cellobiose in the saccharified mash causes inhibition on cellulases decelerating the rate of enzyme hydrolysis (Andric et al., 2010; Lammirato et al., 2010; Shi et al., 2009). Supplementation with cellobiase has been reported to prevent this feedback inhibition (García-Aparicio et al., 2007; Gruno et al., 2004). There are also reports that the extent of xylan removal is directly correlated to cellulose hydrolysis and xylanase supplementation greatly improved cellulose saccharification (Moxley et al., 2012; Zhang et al., 2013; Hu et al., 2011). Yu et al. (2003) observed that the synergism between cellulase and xylanase was due to the ability of the latter to expose cellulose microfibril core by effectively removing hemicelluloses. One of the promising strategies to optimize the fermentable sugar yield from LCBs is the use of well-balanced enzyme cocktails (Zhou et al., 2008). Previous studies on lignocellulo-starch biomass also showed that a full complement of three enzymes such as cellulase, xylanase and starch hydrolyzing enzyme, Stargen could release maximum quantity of fermentable sugars (Mithra et al., 2017a).
Besides the direct binding of lignin to cellulases, most of the lignin-derived phenolic compounds are reported to be inhibitory to cellulases and fermenting organisms (Tejirian and Xu, 2011; Ximenes et al., 2010). The type and concentration of degradation products varied with the pretreatment methods, its severity, temperature, time etc. (Klinke et al., 2002). Surfactants such as Tween and poly ethylene glycol (PEG) have been reported to reduce the level of inhibitors and enhance the saccharification rate (Tejirian and Xu, 2011; Eriksson et al., 2002; Börjesson et al., 2007). The positive effect of surfactant addition on enzymatic digestibility of LCBs has been attributed to the prevention of the non-productive binding of lignin to cellulase by forming complexes with it, thereby making available free cellulase for hydrolysis (Eriksson et al., 2002; Kristensen et al., 2007). Detoxification using sodium borohydride tremendously improved the fermentation of sugarcane bagasse hydrolysate and the effect was mainly by removing furan aldehydes from the pretreated liquor (Cavka and Jönsson, 2013). Previous studies on steam and DSA pretreated lignocellulo-starch biomass showed that Tween 20 and its combination with PEG 4000 could remove 70–81% and 73–82% respectively of phenolic compounds from the pretreated liquor, while sodium borohydride removed up to 50% (Mithra and Padmaja, 2016b). Cost of enzyme being a major barrier to the cost-effective production of second generation (2G) ethanol (Lynd et al., 2008), different strategies are to be explored to reduce enzyme costs. The present study aimed at two cost reduction strategies for enzymatic saccharification of LCSBs such as (i) reducing enzyme dosage through modification of their levels in enzyme cocktails and (ii) enhancing the fermentable sugar yield in enzyme-reduced saccharification systems using detoxification chemicals such as Tween 20, PEG 4000 and sodium borohydride.
2. Materials and methods
2.1. Materials
Peels from root crops such as sweet potato (SP), elephant foot yam (EFY), tannia, greater yam (GY) and beetroot (BR) and vegetables such as pumpkin (PK) and vegetable banana (VB) were collected by manually peeling the samples. Adhering dirt and sand were removed by thorough washing in running tap water and samples were drained and immediately dried in the sun for 36–48 h. The dry samples were powdered to particles of ca 2–3-mm size, and the unscreened powder was then subjected to two pretreatments such as conventional DSA pretreatment and microwave-assisted DSA pretreatment as described under Section 2.3. It was reported earlier that the root crop residues contained cellulose (13–19%), hemicellulose (13–20%), starch (27–32%) and lignin (4–8%), while the vegetable residues contained cellulose (21–22%), hemicellulose (15–17%), starch (25–36%) and lignin (10%) on dry basis (Mithra and Padmaja, 2016a; 2017; Table 1).
Table 1.
Compositional Profile* of The Selected Root and Vegetable Processing Residues (expressed as g/100 g dry basis).
| Parameters | SP peel | EFY peel | Tannia peel | GY peel | BR peel | VB peel | PK peel |
|---|---|---|---|---|---|---|---|
| Cellulose | 13.31 ± 0.03 | 15.63 ± 0.20 | 17.32 ± 0.34 | 18.02 ± 0.58 | 18.94 ± 0.20 | 22.40 ± 0.64 | 21.05 ± 0.79 |
| Hemicellulose | 13.32 ± 0.14 | 14.00 ± 0.00 | 14.48 ± 0.35 | 20.02 ± 0.57 | 19.17 ± 0.55 | 15.19 ± 0.56 | 17.74 ± 0.47 |
| Starch | 32.05 ± 0.09 | 28.96 ± 0.42 | 30.46 ± 0.37 | 28.84 ± 0.44 | 27.13 ± 0.00 | 36.56 ± 0.00 | 24.61 ± 0.00 |
| Lignin | 8.15 ± 0.43 | 7.01 ± 0.13 | 8. 26 ± 0.11 | 6.72 ± 0.17 | 3.87 ± 0.34 | 10.55 ± 0.33 | 10.66 ± 0.84 |
| Total sugars | 11.21 ± 0.01 | 5.53 ± 0.05 | 2.42 ± 0.05 | 4.33 ± 0.00 | 17.07 ± 0.12 | 2.77 ± 0.01 | 8.73 ± 0.06 |
| Reducing sugars | 6.22 ± 0.03 | 2.58 ± 0.00 | 1.34 ± 0.00 | 2.17 ± 0.00 | 6.91 ± 0.04 | 1.71 ± 0.00 | 6.50 ± 0.00 |
| Ash | 3.77 ±0.15 | 9.67 ± 0.12 | 5.27 ± 0.31 | 3.29 ± 0.24 | 5.66 ± 0.10 | 3.40 ± 0.08 | 4.22 ± 0.06 |
| Others** | 18.19 | 19.20 | 21.79 | 18.78 | 8.16 | 9.13 | 13.00 |
Mean ± SD from three replicate.
2.2. Enzymes
Three enzymes such as Ecozyme RT80 (cellulolytic enzyme complex), Ecozyme XY50 (Xylanase) and Stargen™002 (granular starch-hydrolysing enzyme) were used for the study. The former two enzymes were supplied by M/s Ecostar Ltd., Chennai, India, and Stargen was gifted by M/s Genencor International Inc., USA (presently Danisco US Inc., USA). The commercial enzyme mix, Ecozyme RT80 was earlier reported to contain 22 FPU cellulase activity per millilitre besides 328 units of β-glucosidase activity per millilitre (1 unit = mg glucose released per g cellobiose per hour) and 126 units of α- amylase activity per millilitre (1 unit = mg glucose released per g starch per hour) (Mithra et al., 2017a). Stargen™002 contained Aspergillus kawachi α-amylase (E.C. 3.2.1.1) expressed in Trichoderma reesei and a glucoamylase (E.C. 3.2.1.3) from Trichoderma reesei that work synergistically to hydrolyse granular starch substrate to glucose. It has an activity of 570 glucoamylase units (GAU) per gram, and one GAU is the amount of enzyme that will liberate 1 g of reducing sugars (as glucose) per hour from soluble starch substrate under the conditions of the assay (Anon., 2009). Ecozyme RT80, Stargen and Ecozyme XY50 contained 78.8, 216.0 and 5.25 mg crude protein per millilitre respectively (Mithra et al., 2017a).
2.3. Pretreatment techniques
Two of the best pretreatment systems from the previous studies such as conventional dilute sulphuric acid (DSA) pretreatment (121 °C, 60 min, 0.1 mol/L H2SO4) and MW-assisted DSA pretreatment (600 W MW power, 7 min irradiation time, 0.1 mol/L H2SO4) were used for the study (Mithra and Padmaja, 2016a; 2017b). In conventional DSA pretreatment, the biomass powders (10 g) were treated with 100 ml 0.1 mol/L H2SO4 and exposed to heat in a pressure cooker (M/s TTK Prestige India Ltd., India) for 60 min at 121 °C and pressure of 0.102 MPa (as separate lots and time noted after pressure build-up). The flasks after pH adjustment to 5.0 were cooled, and the volume was increased to 100 mL. The pretreated slurries were used for the enzymatic saccharification studies.
In the microwave-assisted DSA pretreatment, the dry biomass powders were suspended in 0.1 mol/L H2SO4 (10% w/v) in Erlenmeyer flasks (250 mL capacity) and proofed at room temperature (30 ± 1 °C) for ten minutes. The slurries were exposed to microwave irradiation power of 600 W and irradiation time of 7 min. in a Microwave oven (M/s Samsung, Thailand) as optimized in a previous study (Mithra et al., 2017b). The slurry was then immediately adjusted to pH 5.0 and treated with the detoxification chemicals as described under Section 2.5.
2.4. Saccharification of conventional DSA pretreated slurry with reduced enzyme dosage
The conventional DSA pretreated slurry after pH adjustment to 5.0 and volume increase to 100 mL was equilibrated in a thermostatic water bath ((M/s Julabo Industries, Germany) at 50 °C for 10 min. Sodium azide (0.25% w/w) was added to prevent microbial growth during saccharification. Three replicates were kept for each biomass and a uniform shaking speed of 150 rpm was ensured throughout the study. The levels of the triple enzyme cocktail standardized earlier (Mithra et al., 2017a) were Ecozyme RT80 [16 FPU/g cellulose), Ecozyme XY50 (3.0 mg protein/g hemicellulose) and Stargen (25 μl equivalent to 5.4 mg protein/g biomass) and the dosages for the latter two enzymes were decreased to either one-half or one-fourth in this study. An enzyme cocktail containing Ecozyme RT80 (16 FPU/g cellulose), Ecozyme XY50 (1.5 mg/g hemicellulose) and Stargen (12.5 μl equivalent to 2.7 mg protein/g biomass) were added for the first treatment (T1). In the case of the second treatment (T2), Ecozyme XY50 and Stargen dosages were reduced to quarter amounts (0.75 mg protein/g hemicellulose for Ecozyme XY50 and 6.25 μl equivalent to 13.5 mg protein/g biomass for Stargen). Samples were incubated for 120 h with pH adjustment to maintain a pH of 5.0 throughout the study. Aliquots were drawn every 24 h to assay the reducing sugar (RS) by the arsenomolybdate reagent (Nelson, 1944). Enzyme blanks as well as substrate blanks were kept during the assay of RS in order to nullify the interference from sugars already present in the commercial enzyme samples and original biomass respectively.
2.5. Effect of supplementing the saccharification system with detoxification chemicals on RS yield
The effect of supplementing the conventional DSA pretreated and MW-assisted DSA pretreated systems with a mixture of detoxification chemicals comprising Tween 20, polyethylene glycol (PEG 4000) and sodium borohydride (NaBH4) on the fermentable sugar yield from enzyme-reduced saccharification system was investigated. The dose level selected for the three detoxification chemicals were based on an earlier study (Mithra and Padmaja, 2016b). The chemical mix having Tween 20 (0.25 v/v), PEG (0.25% w/v) and sodium borohydride (40 mM) were added to the pretreated slurries and kept at room temperature (30 ± 1 °C) for 30 min with occasional shaking. The slurries after pH adjustment to 5.0 and sodium azide addition (0.25% w/w) were equilibrated in a thermostatic water bath at 50 °C for 10 min. The enzyme mix comprising full dose of Ecozyme RT80 (16 FPU/g cellulose), and half dose each of Ecozyme XY50 (1.5 mg protein/g hemicellulose) and Stargen (12.5 μl equivalent to 13.5 mg protein/g biomass) were added to the conventional DSA pretreated slurry and incubation continued up to 120 h (T3).
Based on the previous studies (Mithra et al., 2017b), the enzyme application mode was modified for the MW-assisted DSA pretreated samples as full dose of Ecozyme RT80 (16 FPU/g cellulose) and half dose of Stargen (12.5 μl equivalent to 13.5 mg protein/g biomass) with no Ecozyme XY50 (xylanase) in the present study, as more than 88% hemicellulose was hydrolysed at the pretreatment stage itself. The chemical mix with the same composition as above was added and other saccharification conditions were maintained as described above. A uniform shaking speed of 150 rpm was applied throughout the saccharification for both the saccharification systems and sampling for RS assay was done at 120 h (T4). Three replicates were kept for each biomass and RS was quantified as described earlier (Nelson, 1944).
2.6. Saccharification efficiency and overall conversion efficiency
Saccharification Efficiency (SE %) and Overall Conversion Efficiency (OCE%) were computed from the final reducing sugar yield at 120 h using the following equations for the various systems as reported earlier (Mithra and Padmaja, 2016a):
| SE (%) = ([RSsm – RSp]× 100)/([C + HC + Starch + total sugars] in the original biomass) (1) |
| OCE (%) = (RSsm × 100)/([C + HC + Starch + total sugars] in the original biomass) (2) |
where RSsm = reducing sugar in the 120 h saccharified mash; RSp = reducing sugar in the pretreated liquor; [C+HC+Starch+total sugars] represents the potential sugar yielding carbohydrate fraction in each biomass, comprising cellulose, hemicellulose, starch and total sugars.
2.7. HPLC characterization of sugars
Sugar profile was analysed only for the treatments T3 [conventional DSA pretreated residues saccharified using Stargen and Ecozyme XY50 at half dose and Ecozyme RT80 full dose (T1) + detoxification mix (0.25% Tween 20 + 0.25% PEG + 40 mM NaBH4)] and T4 [MW (600 W)-assisted DSA pretreatment for 7 min + detoxifying chemicals] which proved out to be the best in terms of the Overall Conversion Efficiency and need only be carried forward to the fermentation stage. The saccharified mash (120 h) was centrifuged at 8000 rpm for 10 min. and the clear supernatant was stored at − 4 °C until use. At the time of assay, the filtrates were again filtered through 0.2 μm sterile filters and used for the HPLC characterization of sugars. Analysis of monomeric sugars was performed on an isocratic mode using HPLC (M/s Shimadzu, Kyoto, Japan) and the conditions were: Column: SUPELCOSIL LC-NH2 (25 cm x 4.6 mm), mobile phase: acetonitrile:water (75:25), flow rate: 1.0 ml/min; column temperature: ambient (30 ± 1C); Detector: RID–10 A; injection volume: 20 μl and run time: 30 min.
2.8. Statistical analysis
The data from three replicates were subjected to Analysis of Variance (ANOVA) for statistical testing of the mean values and was followed by least significant difference (LSD) for pair-wise comparison of mean values by using the statistical package, SAS 9.3 (SAS, 2010).
3. Results and discussion
3.1. Effect of reduced enzyme loading on RS release from conventional DSA pretreated lignocellulo-starch biomass
The fermentable sugar yield from conventional DSA pretreated biomass during saccharification (24–120 h) using the two enzyme-reduced systems (T1 and T2) was compared and the decrease in RS in both the systems when compared with the full complement of enzymes reported earlier (Mithra et al., 2017a) was also computed. There was a significant reduction in RS release when Ecozyme XY50 (xylanase) and Stargen doses were reduced to one-fourth the original dose (T2) compared to the system where these doses were only halved (T1) (Table 2). Rapid hydrolysis of polysaccharides was observed during the initial 24 h of saccharification in all the biomass samples, which then decelerated from 24–72 h. The release in RS was only 3–5 g/L RS from both T1 and T2 up to 72 h, irrespective of the dose reduction in enzymes in T2. This indicated that this phase of saccharification was mostly restricted to cellulase action and the rate was unaffected even though the doses of Stargen and xylanase were decreased. The initial RS release might have occurred from the action of xylanase on residual hemicelluloses thereby exposing cellulose for cellulolytic hydrolysis. It was earlier reported that conventional DSA pretreatment for 60 min hydrolysed ca. 42–46% hemicelluloses, 85–94% starch and 3.5- 15% cellulose (Mithra and Padmaja, 2016a). The slowing down of RS release during 24–72 h might have resulted from the feedback inhibition of cellulase by cellobiose (Andric et al., 2010; Lammirato et al., 2010). Ecozyme RT80 used in the present study had 328 units of β-glucosidase activity/ml (Mithra et al., 2017a) which might not have been sufficient to overcome the cellobiose inhibition. The highly significant decrease in RS release from T2 (120 h) indicated that quarter doses of Ecozyme XY50 and Stargen were insufficient (Table 2) to complete the hydrolysis of xylan and starch respectively. Incomplete hydrolysis of xylan during pretreatment has been reported to enhance the level of xylo-oligomers in the pretreated liquor which exert greater inhibition on cellulases than the phenolic compounds released from lignin (Qing et al., 2010).
Table 2.
Reducing sugars released from conventional DSA pretreated processing residues during saccharification with different enzyme loading rates.
| Pretreatment | Reducing sugars in the saccharified mash (g/L) |
||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| (a) SP peel (43.33)* | |||||
| T1 | 49.24± 0.64 (3.24) | 49.72 ± 0.92 (4.12) | 52.59 ± 0.27 (3.58) | 59.82 ± 0.49 (3.01) | 59.89± 0.63 (2.98) |
| T2 | 47.74 ± 0.47 (4.74) | 49.30 ± 0.61 (4.53) | 51.99 ± 0.54 (4.18) | 55.29 ±0.42 (7.54) | 56.25 ± 0.52 (6.62) |
| (b) EFY peel (29.26) | |||||
| T1 | 33.42± 0.55 (5.66) | 35.77 ± 0.72 (4.56) | 37.91 ± 0.43 (4.55) | 45.17 ± 0.46 (3.84) | 45.67 ± 0.48 (3.41) |
| T2 | 31.79 ± 0.74 (7.29) | 33.66 ± 0.97 (6.67) | 36.02 ± 0.44 (6.44) | 39.92 ± 0.57 (9.09) | 40.77 ± 0.61 (8.31) |
| (c) Tannia peel (35.82) | |||||
| T1 | 41.78 ± 0.64 (2.37) | 42.67 ± 0.64 (2.63) | 45.54 ±0.48 (2.29) | 47.84 ± 0.73 (2.64) | 47.96 ± 0.54 (2.60) |
| T2 | 40.09 ± 0.77 (4.06) | 41.72 ± 0.47 (3.58) | 43.82 ± 0.69 (4.01) | 46.92 ± 0.55 (3.56) | 47.04 ± 0.61 (3.52) |
| (d) GY peel (39.48) | |||||
| T1 | 45.90 ± 0.57 (2.96) | 47.03 ± 0.54 (3.58) | 51.02 ± 0.73 (2.38) | 58.63 ± 0.64 (1.29) | 59.01 ± 0.44 (0.95) |
| T2 | 42.88 ± 0.77 (5.98) | 46.42 ± 0.61 (4.19) | 49.00 ± 0.84 (4.40) | 58.22 ± 0.54 (1.70) | 58.44 ± 0.72 (1.52) |
| (e) BR peel (43.00) | |||||
| T1 | 53.09 ± 0.91 (2.39) | 53.69 ± 0.88 (3.03) | 56.36 ± 0.83 (2.60) | 64.66 ± 0.52 (1.03) | 64.77 ± 0.61 (0.99) |
| T2 | 45.94 ± 0.87 (9.54) | 48.95 ± 0.73 (7.77) | 50.98 ± 0.42 (7.98) | 60.09 ± 0.73 (5.60) | 61.19 ± 0.54 (4.57) |
| (f) PK peel (40.56) | |||||
| T1 | 48.77 ± 0.54 (3.01) | 49.49 ± 0.74 (3.56) | 52.47 ± 0.57 (2.99) | 59.85 ± 0.31 (2.35) | 60.26 ± 0.77 (1.98) |
| T2 | 43.53 ± 0.58 (8.25) | 46.75 ± 0.88 (6.30) | 49.08 ± 0.64 (6.38) | 58.11 ± 0.47 (4.09) | 59.81 ± 0.64 (2.43) |
| (g) VB peel (45.00) | |||||
| T1 | 50.72 ± 0.31 (3.25) | 51.77 ± 0.73 (3.58) | 55.01 ± 0.27 (2.69) | 61.94 ± 0.63 (2.46) | 62.86 ± 0.87 (1.57) |
| T2 | 48.62 ± 0.77 (5.35) | 50.15 ± 0.72 (5.20) | 52.25 ± 0.84 (5.45) | 57.15 ± 0.91 (7.25) | 58.35 ± 0.76 (6.08) |
Initial content of sugars in the pretreated liquor; figures in parentheses in each column indicate the decrease in RS compared to the system with full complement of the three enzymes (Mithra et al., 2017a); T1: Stargen and Ecozyme XY 50 half dose and Ecozyme RT80 full dose; T2: Stargen and Ecozyme XY 50 1/4thdose and Ecozyme RT 80 full dose. Each value is mean ± SD from three replicates.
The rapid increase in RS release during 72–96 h of saccharification might have been contributed by Stargen-aided hydrolysis of starch along with cellulase action. Tapering off RS release after 96 h indicated the possibility of feedback inhibition of all the complementary enzymes by monomeric sugars. Such a biphasic pattern of sugar release was reported from these biomass residues saccharified with full complement of enzymes as well (Mithra et al., 2017b). It was further observed that the decrease in RS release at 120 h saccharified mash from T1 was only 0.95–3.41 g/L compared to the system having full complement of enzymes (Table 2). It was reported earlier that Ecozyme RT80 also contained 126 units of α-amylase (Mithra et al., 2017a) and this along with Stargen could facilitate almost complete hydrolysis of starch despite reducing the Stargen levels to half. This is evident from the high RS content in T1 in the various LCSBs after 120 h saccharification. The decrease in RS in 120 h saccharified hydrolysate from T1 was negligible in the case of residues such as GY and BR peels, when compared to the system with full complement of enzymes (Mithra et al., 2017a), whereas 2.6–3.4 g/L decrease was observed in RS content in SP, EFY and tannia peels (Table 2), which indicated that the extent of deconstruction at the pretreatment stage also plays a role in the final saccharification yield. Although the RS content in EFY and tannia peel hydrolysates appeared to be significantly lower than the other residues, it could be seen that this was not due to poor saccharification but had resulted from the low initial levels of RS in the pretreated liquor (29.26 g/L and 35.82 g/L in EFY and tannia peel hydrolysates respectively). Among the residues, the highest saccharification rate was observed in BR peel, which might have resulted from the lowest lignin content (3.8 g/100 g) in this residue (Mithra et al., 2017a). Jing et al. (2009) also reported that lignin degradation products exerted significant inhibition effect on cellulase enzymes.
Response surface optimization studies conducted earlier showed that the optimum dose of Ecozyme RT80 was 16 FPU/g cellulose and hence this dose level was maintained in all the treatments in the present study as well. Kumar and Wyman (2009) found that xylanase leverage on glucan removal decreased at high loading levels of cellulase and the findings from the present study supports the report mainly because the commercial cellulase preparations contain xylanase also as co- activity. Garcia-Aparicio et al. (2007) also reported that although the commercial cellulolytic enzymes contained β-glucosidase or xylanase, these levels were not often sufficient to facilitate the hydrolysis of polysaccharides. The complementary role of both Stargen and xylanase in enhancing fermentable sugar yield from steam pretreated LCSBs was established earlier (Mithra et al., 2017a).
3.2. Effect of supplementation with detoxifying chemicals on RS yield
Lignin degradation products (phenolics) are reported to be highly inhibitory to cellulases (Larsson et al., 2000; Tejirian and Xu, 2011; Ximenes et al., 2010). Hence the effect of supplementing the saccharification systems such as conventional DSA pretreated system and MW-assisted DSA pretreated system with detoxification chemicals such as Tween 20, Polyethylene glycol (PEG 4000) and sodium borohydride on enhancing fermentable sugar yield was studied. In the case of conventional DSA pretreatment, T1 (full dose of Ezozyme RT80 and half dose each of Stargen and Ecozyme XY 50) was supplemented with the detoxification chemical mix (0.25% v/v Tween 20+0.25% w/v PEG+40 mM sodium borohydride). Time course release of sugars during 24–120 h from this system (T3) indicated that there was significant increase in RS release compared to T1 in all the LCSBs (Table 3 vs Table 2). There was only a negligible decrease of 0.43–1.85 g/L RS release in the system supplemented with detoxification chemicals also in combination with reduced enzyme dosage (T3) compared to system having full complement of enzymes (Table 3). This was evidently due to the reversion of inhibition of enzymes by the detoxification chemicals. Except in the case of pumpkin peel, there was maximum release of RS at 96 h of saccharification itself and as in the case of the system without detoxification chemicals (T1), highest RS values were obtained in the hydrolysates from BR (65.33 g/L) and VB (63.88 g/L) peels. Previous studies showed that phenolic compounds were generated during pretreatment of LCSBs and the three chemicals had significant effect in reducing their levels in the pretreated liquor (Mithra and Padmaja, 2016b). These effective levels of the inhibitors were used in the present study as well. The levels of RS achieved in 120 h saccharified mash from DSA pretreated system supplemented with the detoxification chemicals were almost similar to those obtained with the full complement of the three enzymes reported before by Mithra et al. (2017a) indicating that considerable saving of enzymes such as Stargen and xylanase (Ecozyme XY50) was possible when surfactants and sodium borohydride were also supplemented.
Table 3.
Time course release of reducing sugars from conventional DSA pretreated processing residues along with detoxifying chemicals (T3)*.
| Biomass | Reducing sugars in the saccharified mash (g/L) |
||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| SP peel | 51.09c | 51.11c | 54.84b | 61.33a | 61.02a (1.85) |
| EFY peel | 35.66d | 37.88c | 39.23b | 47.21a | 47.63a (1.45) |
| Tannia peel | 43.09c | 44.06c | 46.08b | 48.48a | 49.01a (1.55) |
| GY peel | 46.77d | 48.41c | 52.03b | 58.91a | 59.33a (0.63) |
| BR peel | 54.21c | 55.22c | 57.12b | 65.07a | 65.33a (0.43) |
| PK peel | 49.09e | 51.42d | 54.08c | 60.11b | 61.42a (0.82) |
| VB peel | 51.23d | 53.01c | 56.77b | 63.21a | 63.88a (0.55) |
T3: T1 (Stargen and Ecozyme XY 50 half dose and Ecozyme RT80 full dose) along with detoxification mix (0.25% Tween 20+ 0.25% PEG+ 40 mM NaBH4); figures in parentheses indicate the decrease in RS compared to the system with full complement of the three enzymes (Mithra et al., 2017a); statistical comparison was made within each row and values with different superscripts are significant at p < 0.05.
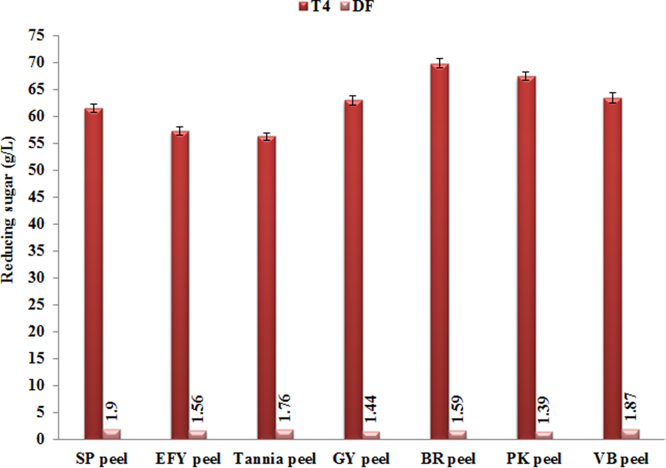
Supplementation of the MW-assisted DSA pretreated system saccharified using only two enzymes such as Ecozyme RT80 and Stargen (full dose for the former and half dose for the latter) with detoxification chemicals (T4) had significant effect in enhancing RS yield almost comparable to the MW system saccharified with the full complement of three enzymes, with only a decrease of 1.4–1.9 g/L (DF; Fig. 1). It was earlier reported that the MW-assisted DSA pretreatment under optimized conditions was very effective in enhancing RS yield from the LCSBs (Mithra et al., 2017b). Xylanase was not supplemented to the enzyme-reduced system in the present study due to the very high hydrolysis of hemicelluloses (ca. 88%) at the pretreatment stage itself as reported by Mithra et al. (2017b). The RS content in the MW-assisted DSA pretreated system saccharified with only Ecozyme RT80 and Stargen was higher (56.2–69.8 g/L) than the DSA pretretaed system saccharified with triple enzymes indicating the comparative dual advantage of enzyme saving and short pretreatment time (7 min) in the former. The MW system was especially beneficial for EFY and tannia peels with a significantly higher RS release which also had the lowest hemicelluloses content (14%; Mithra and Padmaja, 2016a). Kumar and Wyman (2009) also reported that incubation of dilute acid pretreated solids with Tween 20 or PEG 6000 prior to adding enzymes enhanced the RS yield. Qi et al. (2010) found that non-ionic surfactants could enhance the enzymatic conversion of cellulose to sugars and also lower the amount of cellulolytic enzymes required to obtain higher yield. Inhibition of cellulases by phenols have been reported by several workers (Tejirian and Xu, 2011; Ximenes et al., 2010). Besides cellulase, β-glucosidase and xylanase were also reported to be inhibited by lignin preparations (Berlin et al., 2006). Gao et al. (2014) reported the irreversible loss of cellulase by lignin during saccharification, stressing the need to channel out lignin, prior to saccharification.
Fig. 1.
Reducing sugar yield from MW-assisted DSA pretreated biomass saccharified using Ecozyme RT80 (full dose) and Stargen (half dose) along with detoxifying chemicals (T4). Line 2 (DF) gives the decrease in reducing sugars released from T4 compared to full complement of enzymes.
3.3. Saccharification efficiency (SE) and overall conversion efficiency (OCE)
A comparison of Saccharification Efficiency for the various systems is presented in Table 4 which indicated that the MW-assisted DSA pretreated biomass saccharified using full dose of Ecozyme RT80 and half dose of Stargen without xylanase and supplemented with the detoxifying chemicals (T4) had the highest saccharification efficiency. There was a drastic improvement in SE (%) in T4 compared to T3 where enzyme-reduced saccharification of DSA pretreated biomass was conducted with full dose of Ecozyme RT80 along with half dose each of Stargen and Ecozyme XY50 in combination with detoxification chemicals. Variation in the SE depending on the type of biomass was evident with only negligible difference between T1 and T3 in peels from greater yam, beetroot and pumpkin (Table 4). When compared to the conventional DSA pretreatment requiring 60 min duration, MW-assisted DSA pretreatment was advantageous due to the short processing time (7 min), which may also restrict the chances of formation of high quantities of inhibitors. The high hemicellulose hydrolysis (ca. 88%) reported in MW-assisted DSA pretreatment (Mithra et al., 2017b) could also justify the elimination of xylanase from the saccharification step.
Table 4.
Saccharification Efficiency (%) in conventional and MW-assisted DSA pretreated lignocellulo-starch biomass: effect of enzyme loading rates and detoxification chemicals.
| Biomass | SE (%) |
|||
|---|---|---|---|---|
| Conventional DSA pretreatment* and saccharification |
Microwave-assisted DSA pretreatment and saccharification |
|||
| T1 | T2 | T3 | T4 | |
| SP peel | 23.69c | 18.49d | 25.31b | 33.11a |
| EFY peel | 25.59c | 17.95d | 28.65b | 33.00a |
| Tannia peel | 18.77c | 17.34d | 20.39b | 30.10a |
| GY peel | 27.42bc | 26.63c | 27.88b | 29.27a |
| BR peel | 26.46b | 22.10c | 27.13b | 28.89a |
| PK peel | 27.31bc | 26.68c | 28.92b | 31.51a |
| VB peel | 23.22c | 17.35d | 24.54b | 26.00a |
Conventional DSA pretreatment (121 °C; 60 min; 0.1 M H2SO4); T1: Stargen and Ecozyme XY 50 half dose and Ecozyme RT80 full dose; T2: Stargen and Ecozyme XY 50 1/4thdose and Ecozyme RT 80 full dose; T3 is T1 + detoxifying chemicals (0.25% Tween 20+ 0.25% PEG+ 40 mM NaBH4); T4 is MW(600 W)-assisted DSA pretreatment (7 min) with detoxifying chemicals; statistical comparison was made within each row and values with different superscripts are significant at p < 0.05.
The Overall Conversion Efficiency of carbohydrates to fermentable sugars was maximum for the MW-assisted DSA pretreatment system (T4) saccharified using Ecozyme RT80 (full dose) and Stargen (half dose) along with the detoxification chemicals. The percentage reduction in OCE compared to the conventional DSA pretreated system saccharified using full complement of enzymes ranged from 1.20–5.31% in T1 and 2.13–12.96% in T2 indicating that reducing the levels of Stargen and Ecozyme XY50 to 1/4th the original dose (T2) drastically affected OCE (Table 5). It was found that the reduction in OCE obtained in T1 could be overcome to a very high extent by supplementing the system with detoxifying chemicals (T3). The decrease in OCE observed in T3 compared to the system with full complement of enzymes was negligible (0.52–2.65%). As high as 85–93% of carbohydrate could be converted to fermentable sugars in T4 indicating that cost saving through reduction in enzyme dosage of Stargen and eliminating xylanase (Ecozyme XY50) was possible. Approximately 1.9–2.7% reduction in OCE was noticed in T4 when compared to similar system saccharified with the full complement of the three enzymes, without detoxification chemicals. There was phenomenal increase in T4 OCE in residues such as peels from EFY, tannia and pumpkin compared to T1 OCE, which indicated that the deconstruction of these residues was better in MW-assisted DSA pretreatment than in conventional DSA pretreatment. Bussamra et al. (2015) also observed that a designer cocktail of Trichoderma reesei fraction (80%), endoglucanase (10%) and β-glucosidase (10%) could convert 72% of the cellulose to sugars in sugarcane bagasse, indicating only small quantities of the latter two enzymes were sufficient. As different from the lignocellulosic biomass containing only cellulose and hemicellulose as polysaccharides, lignocellulo-starch biomass (peels of roots and vegetables) also contain lot of starch which necessitates whole slurry saccharification. Hence soluble lignin degradation products present in the pretreated liquor as well as unhydrolysed lignin remaining in the residue have to be effectively removed to achieve high fermentable sugar yield. The high OCE obtained in the present study proved that the detoxification chemicals at the levels used were effective.
Table 5.
Overall Conversion Efficiency (%) in conventional and MW-assisted DSA pretreated lignocellulo-starch biomass: effect of enzyme loading rates and detoxification chemicals*.
| Biomass | OCE (%) |
|||
|---|---|---|---|---|
| Conventional DSA pretreatment and saccharification |
MW-assisted DSA pretreatment and saccharification |
|||
| T1 | T2 | T3 | T4 | |
| SP peel | 85.69b (4.27) | 80.48c (9.48) | 87.31a (2.65) | 87.97a (2.72) |
| EFY peel | 71.23c (5.31) | 63.58d (12.96) | 74.28 b (0.88) | 89.21a (2.43) |
| Tannia peel | 74.14c (4.02) | 72.72d (5.44) | 75.76 b (1.88) | 86.84a (2.73) |
| GY peel | 82.87bc (1.33) | 82.07c (2.13) | 83.32b (0.88) | 88.37a (2.02) |
| BR peel | 78.70b (1.2) | 74.35c (5.55) | 79.38b (0.52) | 84.78a (1.93) |
| PK peel | 83.53c (2.75) | 82.91c (3.37) | 85.14b (1.14) | 93.44a (1.93) |
| VB peel | 81.71b (2.04) | 75.85c (7.90) | 83.04a (0.71) | 82.48a (2.43) |
3.4. HPLC profile of sugars
The monomeric sugar profile of the enzyme saccharified hydrolysate from T3 and T4 is given in Table 6. Although glucose was uniformly present in all the hydrolysates, there was preponderance of it in beet root peel (BR peel) hydrolysates. Highest value of xylose was obtained for tannia peel and GY peel and VB peels. Arabinose was present only in SP peel hydrolysates, while galactose was present in BR peel and PK peel hydrolysates only which indicated the difference in the sugar composition of hemicellulose in the various peel samples. Mannose could not be detected in the hydrolysate from SP peel and PK peel. A similar profile was obtained for the MW-assisted DSA pretreated and saccharified samples also with slightly higher amounts of the various sugars (Table 6). The study indicated that co-fermentation with hexose and pentose fermenting yeasts may be significant in the case of hydrolysates other than that from BR peel because of moderate to reasonably high levels of xylose in them.
Table 6.
HPLC sugar profile in the hydrolysates from conventional and MW-assisted DSA pretreated and sachharified lignocellulo-starch biomass.
| Type of sugars | Sugar content (g/L) |
||||||
|---|---|---|---|---|---|---|---|
| SP peel | EFY peel | Tannia peel | GY peel | BR peel | PK peel | VB peel | |
| (a) Conventional DSA pretreatment (T3) and saccharification | |||||||
| Glucose | 33.41 | 21.33 | 19.55 | 27.31 | 40.36 | 33.21 | 29.65 |
| Xylose | 4.23 | 9.55 | 11.64 | 14.69 | 1.96 | 8.61 | 11.40 |
| Mannose | ND | 1.47 | 3.69 | 5.64 | 2.91 | ND | 7.36 |
| Arabinose | 9.71 | ND | ND | ND | ND | ND | ND |
| Galactose | ND | ND | ND | ND | 5.64 | 1.36 | ND |
| (b) MW-assisted DSA pretreatment (T4) and saccharification | |||||||
| Glucose | 35.13 | 23.05 | 21.27 | 29.03 | 42.08 | 34.93 | 31.37 |
| Xylose | 5.77 | 11.09 | 13.18 | 15.40 | 3.50 | 10.15 | 12.94 |
| Mannose | ND | 2.41 | 4.63 | 6.58 | 3.85 | ND | 8.30 |
| Arabinose | 10.26 | ND | ND | ND | ND | ND | ND |
| Galactose | ND | ND | ND | ND | 6.88 | 2.60 | ND |
T3: Conventional DSA pretreated residues (121 °C; 60 min; 0.1 M H2SO4) + detoxification chemicals; T4 is MW (600 W)-assisted DSA pretreated residues (7 min) with detoxifying chemicals; Both the systems were saccharified using Stargen and Ecozyme XY50 at half dose and Ecozyme RT80 full dose; ND: not detected; each value represents mean from two runs.
4. Conclusions
The possibility of enzyme saving during saccharification of pretreated lignocellulo-starch biomass was investigated using two optimized pretreatment systems such as conventional DSA pretreatment and microwave-assisted pretreatment and supplementing the systems with detoxification chemicals such as Tween 20, PEG 4000 and sodium borohydride. Detoxification of pretreated slurry prior to saccharification with full dose of Ecozyme RT80 (cellulase) and half doses of Ecozyme XY50 (xylanase) and Stargen could enhance RS release from conventional DSA pretreated (60 min) biomass. Nevertheless significantly higher conversion efficiency was observed in MW-assisted DSA pretreated system with a much shorter processing time (7 min) and without the need for xylanase. HPLC sugar profile indicated the presence of glucose in high amounts in all the hydrolysates with xylose in reasonably good amounts in hydrolysates except BR peel. As cost of enzymes is a major barrier to the production of 2 G ethanol at low cost, the present study opens at the possibility of cost reduction through effective pretreatment such as conventional or MW-assisted DSA pretreatment followed by saccharification with reduced enzyme dosage/effective enzyme combinations in presence of detoxification chemicals.
Declarations
Author contribution statement
M. G. Mithra: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
G. Padmaja: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Kerala State Council for Science, Technology & Environment (KSCSTE) (Grant No. 853/2015/KSCSTE).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the Director, ICAR-CTCRI for the facilities provided. The help extended by Dr. J. Sreekumar, Principal Scientist (Agricultural Statistics) for the statistical analysis is gratefully acknowledged. Stargen™002 was received by courtesy from M/s Genencor International Inc., USA (presently Danisco US Inc., USA).
References
- Andric P., Meyer A.S., Jensen P.A., Dam-Johansen K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol. Adv. 2010;28:308–324. doi: 10.1016/j.biotechadv.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Anon . Product information (2009) published by Genencor International, a Division of Danisco, Danisco US Inc.; 2009. STARGEN™002: Granular starch hydrolyzing enzyme for ethanol production.http//www.genencor.com accessed 22.12.2014. [Google Scholar]
- Benjamin Y., Cheng H., Gorgens J.F. Optimization of dilute sulphuric acid pretreatment to maximize combined sugar yield from sugarcane bagasse for ethanol production. Appl. Biochem. Biotechnol. 2014;172:610–630. doi: 10.1007/s12010-013-0545-z. [DOI] [PubMed] [Google Scholar]
- Berlin A., Balakshin M., Gilkes N., Kadla J., Maximenko V., Kubo S. Inhibition of cellulase, xylanase and β-glucosidase activities by softwood lignin preparations. J. Biotechnol. 2006;125:198–209. doi: 10.1016/j.jbiotec.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Börjesson J., Peterson R., Tjerneld F. Enhanced enzymatic conversion of softwood lignocelluloses by poly (ethylene glycol) addition. Enzyme Microb. Technol. 2007;40:754–762. [Google Scholar]
- Bussamra B.C., Freitas S., da Costa A.C. Improvement on sugarcane bagasse hydrolysis using enzymatic mixture designed cocktail. Bioresour. Technol. 2015;187:173–181. doi: 10.1016/j.biortech.2015.03.117. [DOI] [PubMed] [Google Scholar]
- Cavka A., Jönsson L.J. Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour. Technol. 2013;136:368–376. doi: 10.1016/j.biortech.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Chen H.Z., Liu L.Y. Unpolluted fractionation of wheat straw by steam explosion and ethanol extraction. Bioresour. Technol. 2007;98:666–676. doi: 10.1016/j.biortech.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Eriksson T., Börjesson J., Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol. 2002;31:353–364. [Google Scholar]
- Ethaib S., Omar R., Kamal S.M.M., Biak D.R.A. Microwave-assisted pretreatment of lignocellulosic biomass: a review. J. Eng. Sci. Technol. 2015;21:97–109. [Google Scholar]
- Farrel A.E., Pelvin R.J., Turner B.T., Jones A.D., O’ Hare M., Kammen D.M. Ethanol can contribute to energy and environmental goals. Science. 2006;311:506–508. doi: 10.1126/science.1121416. [DOI] [PubMed] [Google Scholar]
- Gao D., Haarmeyer C., Balan V., Whitehead T.A., Dale B.E., Chundawat P.S. Lignin triggers irreversible cellulase loss during pretreated lignocellulosic biomass saccharification. Biotechnol. Biofuels. 2014;7:1–13. doi: 10.1186/s13068-014-0175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Aparicio M.P., Ballesteros M., Manzanares P., Ballesteros I., González A., Negro M.J. Xylanase contribution to the efficiency of cellulase enzymatic hydrolysis of barley straw. Appl. Biochem. Biotechnol. 2007;137-140:353–365. doi: 10.1007/s12010-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Gruno M., Väljamäe P., Pettersson G., Johansson G. Inhibition of the Trichoderma reesei cellulases by cellobiose is strongly dependent on the nature of the substrate. Biotechnol. Bioeng. 2004;86:503–511. doi: 10.1002/bit.10838. [DOI] [PubMed] [Google Scholar]
- Himmel M.E., Ding S.Y., Johnson D.K., Adney W.S., Nimlos M.R., Brady J.W., Foust T.D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;325:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Hu J., Arantes V., Saddler J.N. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol. Biofuels. 2011;4:1–13. doi: 10.1186/1754-6834-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X., Zhang X., Bao J. Inhibition performance of lignocellulose degradation products on industrial cellulase enzymes during cellulose hydrolysis. Appl. Biochem. Biotechnol. 2009;159:696–707. doi: 10.1007/s12010-009-8525-z. [DOI] [PubMed] [Google Scholar]
- Klinke H.B., Ahring B.A., Schmidt A.S., Thomsen A.B. Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour. Technol. 2002;82:15–26. doi: 10.1016/s0960-8524(01)00152-3. [DOI] [PubMed] [Google Scholar]
- Kristensen J.B., Börjesson J., Bruun M.H., Tjerneld F., Jørgensen H. Use of surface active additives in enzymatic hydrolysis of wheat straw lignocelluloses. Enzyme Microb. Technol. 2007;40:888–895. [Google Scholar]
- Kuhnel S., Schols H.A., Gruppen H. Aiming for the complete utilization of sugarbeet pulp: examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol. Biofuels. 2011;4:14. doi: 10.1186/1754-6834-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Wyman C.E. Effects of cellulase and xylanase on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Prog. 2009;25:302–314. doi: 10.1002/btpr.102. [DOI] [PubMed] [Google Scholar]
- Kurakake M., Ooshima H., Kato J., Harano Y. Pretreatment of bagasse by non-ionic surfactant for the enzymatic hydrolysis. Bioresour. Technol. 1994;49:247–251. [Google Scholar]
- Lammirato C., Miltner A., Wick L.Y., Kästner M. Hydrolysis of cellobiose by β- glucosidase in the presence of soil minerals-interactions at solid-liquid interfaces and effects on enzyme activity levels. Soil Biol. Biochem. 2010;42:2203–2210. [Google Scholar]
- Larsson S., Quintana-Sáinz A., Reimann A., Nilvebrant N.O., Jönsson L.J. Influence of lignocellulose-derived aromatic compounds on oxygen limited growth and ethanolic fermentation by Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2000;84:617–632. doi: 10.1385/abab:84-86:1-9:617. [DOI] [PubMed] [Google Scholar]
- Li A., Antizar-Ladislao B., Khraisheh M. Bioconversion of municipal solid waste to glucose for bio-ethanol production. Biopro. Biosys. Eng. 2007;30:189–196. doi: 10.1007/s00449-007-0114-3. [DOI] [PubMed] [Google Scholar]
- Li H., Qu Y., Yang Y., Chang S., Xu J. Microwave irradiation- a green and efficient way to pretreat biomass. Bioresour. Technol. 2016 doi: 10.1016/j.biortech.2015.08.099. [DOI] [PubMed] [Google Scholar]
- Lissens G., Klinke H., Verstraete W., Ahring B., Thomsen A.B. Wet oxidation treatment of organic household waste enriched with wheat straw for simultaneous saccharification and fermentation into ethanol. Environ. Technol. 2004;25:647–655. doi: 10.1080/09593330.2004.9619354. [DOI] [PubMed] [Google Scholar]
- Lynd L.R., Laser M.S., Brandsby D., Dale B.E., Davison B., Hamilton R. How biotech can transform biofuels. Nature Biotechnol. 2008;26:169–172. doi: 10.1038/nbt0208-169. [DOI] [PubMed] [Google Scholar]
- Mansfield S.D., Mooney C., Saddler J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Appl. Biochem. Biotechnol. 1999;15:804–809. doi: 10.1021/bp9900864. [DOI] [PubMed] [Google Scholar]
- Martin C., Galbe M., Wahlboma C.F., Hahn-Hägerdal B., Jönsson L.J. Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb. Technol. 2002;31:274–282. [Google Scholar]
- Mithra M.G., Padmaja G. Compositional profile and ultrastructure of selected root and vegetable processing residues subjected to steam and dilute sulfuric acid pretreatment. Curr. Biotechnol. 2016;7 [Google Scholar]
- Mithra M.G., Padmaja G. Phenolic inhibitors of saccharification and fermentation in lignocellulo-starch prehydrolysates and comparative efficacy of detoxification treatments. J. Biomass Biofuel. 2016;3:1–15. [Google Scholar]
- Mithra M.G., Sreekumar J., Padmaja G. Binary- and triple-enzyme cocktails and their application mode affect fermentable sugar release from pretreated lignocellulo-starch biomass. Biomass Conv. Biorefin. 2017 [Google Scholar]
- Mithra M.G., Padmaja G., Sreekumar J. Optimization of microwave-assisted dilute acid pretreatment for enhanced structural breakdown and enzymatic saccharification of lignocellulo-starch biomass. Curr. Microwave Chem. 2017;4:1–13. [Google Scholar]
- Mithra M.G., Padmaja G. Comparative alterations in the compositional profile of selected root and vegetable peels subjected to three pretreatments for enhanced saccharification. Internat. J. Environ. Agric. Biotechnol. 2017;2:1732–1744. [Google Scholar]
- Mosier N.S., Hendrickson R., Ho N., Sedlak M., Ladisch M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005;96:1986–1993. doi: 10.1016/j.biortech.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Moxley G., Gaspar A.R., Higgins D., Xu H. Structural changes of corn stover lignin during acid pretreatment. J. Indus. Microbiol. Biotechnol. 2012;39:1289–1299. doi: 10.1007/s10295-012-1131-z. [DOI] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for determination of glucose. J. Biol. Chem. 1944;153:375–380. [Google Scholar]
- Öhgren K.H., Hahn B., Zacchi G. Simultaneous saccharification and co-fermentation of glucose and xylose in steam pretreated corn stover at high fiber content with S. cerevisiae. J. Biotechnol. 2006;126:488–496. doi: 10.1016/j.jbiotec.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Palmqvist E., Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000;74:25–33. [Google Scholar]
- Qi B.K., Chen X.R., Wan Y.H. Pretreatment of wheat straw by non-ionic surfactant-assisted dilute acid for enhancing hydrolysis and ethanol production. Bioresour. Technol. 2010;101:4875–4883. doi: 10.1016/j.biortech.2010.01.063. [DOI] [PubMed] [Google Scholar]
- Qing Q., Yang B., Wyman C.E. Xylo-oligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010;101:9624–9630. doi: 10.1016/j.biortech.2010.06.137. [DOI] [PubMed] [Google Scholar]
- Saha B.C., Cotta M.A. Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. New Biotechnol. 2009;27:10–16. doi: 10.1016/j.nbt.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Ghosh S.K., Bannerjee S., Aikat K. Bioethanol production from agricultural wastes: An overview. Renew. Ener. 2012;37:19–27. [Google Scholar]
- SAS . SAS Institute Inc.; Cary NC, USA: 2010. SAS. [Google Scholar]
- Shi J., Sharma-Shivappa R.R., Chinn M., Howell N. Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioener. 2009;33:88–96. [Google Scholar]
- Singh A., Kuila A., Adak S., Bishai M., Banerjee R. Utilization of vegetable wastes for bioenergy generation. Agric. Res. 2012;1:213–222. [Google Scholar]
- Sun Y., Cheng J.Y. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002;83:1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Tang Y.Q., Koike Y., Liu K., An M.Z., Morimura S., Wu X.L. Ethanol production from kitchen waste using the flocculating yeast, Saccharomyces cerevisiae strain KF-7. Biomass Bioener. 2008;32:1037–1045. [Google Scholar]
- Tejirian A., Xu F. Inhibition of enzymatic cellulolysis by phenolic compounds. Enzyme Microb. Technol. 2011;48:239–247. doi: 10.1016/j.enzmictec.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Wyman C.E. Biomass ethanol: technical progress, opportunities and commercial challenges. Annu. Rev. Energ. Environ. 1999;24:189–226. [Google Scholar]
- Ximenes E.A., Kim Y., Mosier N.S., Dien B.S., Ladisch M.R. Inhibition of cellulases by phenols. Enzyme Microb. Technol. 2010;46:170–176. doi: 10.1016/j.enzmictec.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Yu P., Mckinon J.J., Maenz D.D., Olkowski A.A., Raez V.J., Christensen D.A. Enzymic release of reducing sugars from oat hulls by cellulase, as influenced by Aspergillus ferulic acid esterase and Trichoderma xylanase. J. Agric. Food Chem. 2003;51:218–223. doi: 10.1021/jf020476x. [DOI] [PubMed] [Google Scholar]
- Zhang B., Shahbazi A. Recent developments in pretreatment technologies for production of lignocellulosic biofuels. J. Pet. Environ. Biotechnol. 2011;2:108–115. [Google Scholar]
- Zhang C., Zhuang X.X., Wang Z.J., Matt F., St John F., Zhu J.Y. Xylanase supplementation on enzymatic saccharification of dilute acid pretreated poplars at different severities. Cellulose. 2013;20:1937–1946. [Google Scholar]
- Zhou J., Wang Y.-H., Chu J., Luo L.-Z., Zhuang Y.-P., Zhang S.-L. Optimization of cellulase mixture for efficient hydrolysis of steam-exploded corn stover by statistically designed experiments. Bioresour. Technol. 2008;100:819–825. doi: 10.1016/j.biortech.2008.06.068. [DOI] [PubMed] [Google Scholar]
- Zhu W., Houtman C.J., Zhu J.Y., Gleisner R., Chen K.F. Quantitative predictions of bioconversion of aspen by dilute acid and SPORL pretreatments using a unified combined hydrolysis factor (CHF) Process Biochem. 2012;47:785–791. [Google Scholar]