Abstract
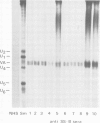
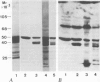
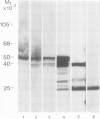
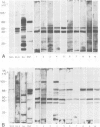
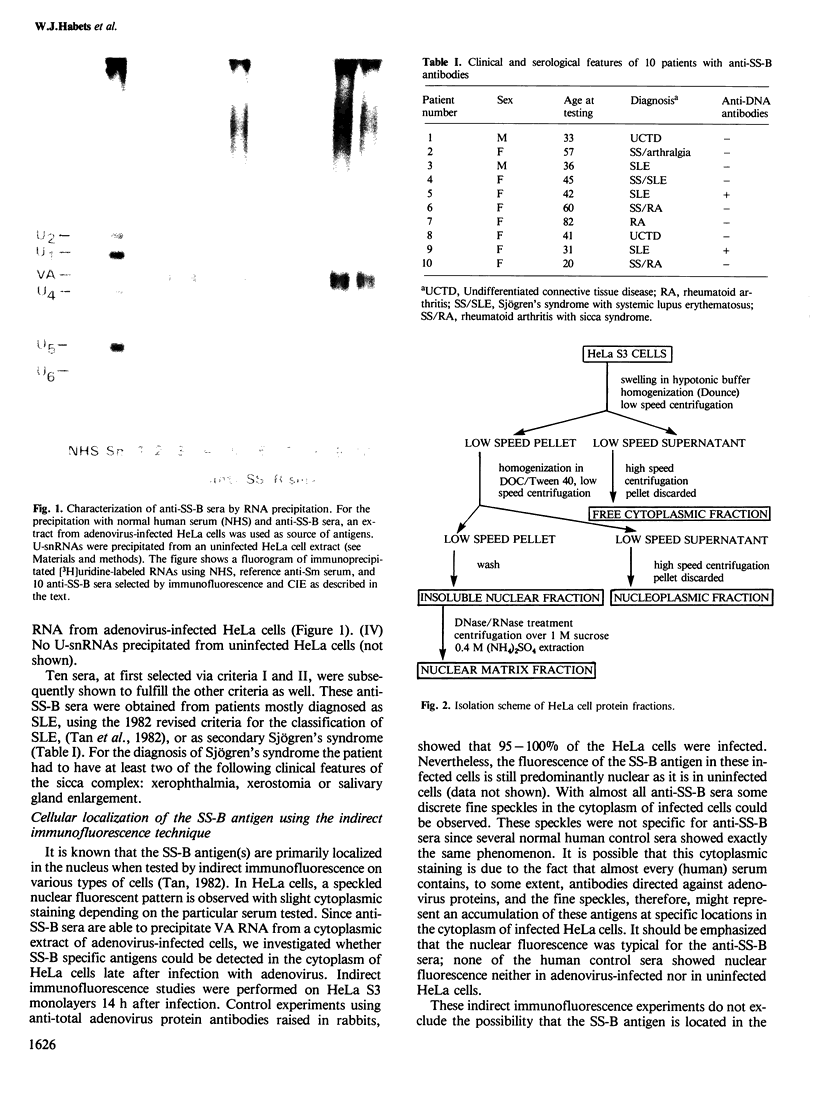
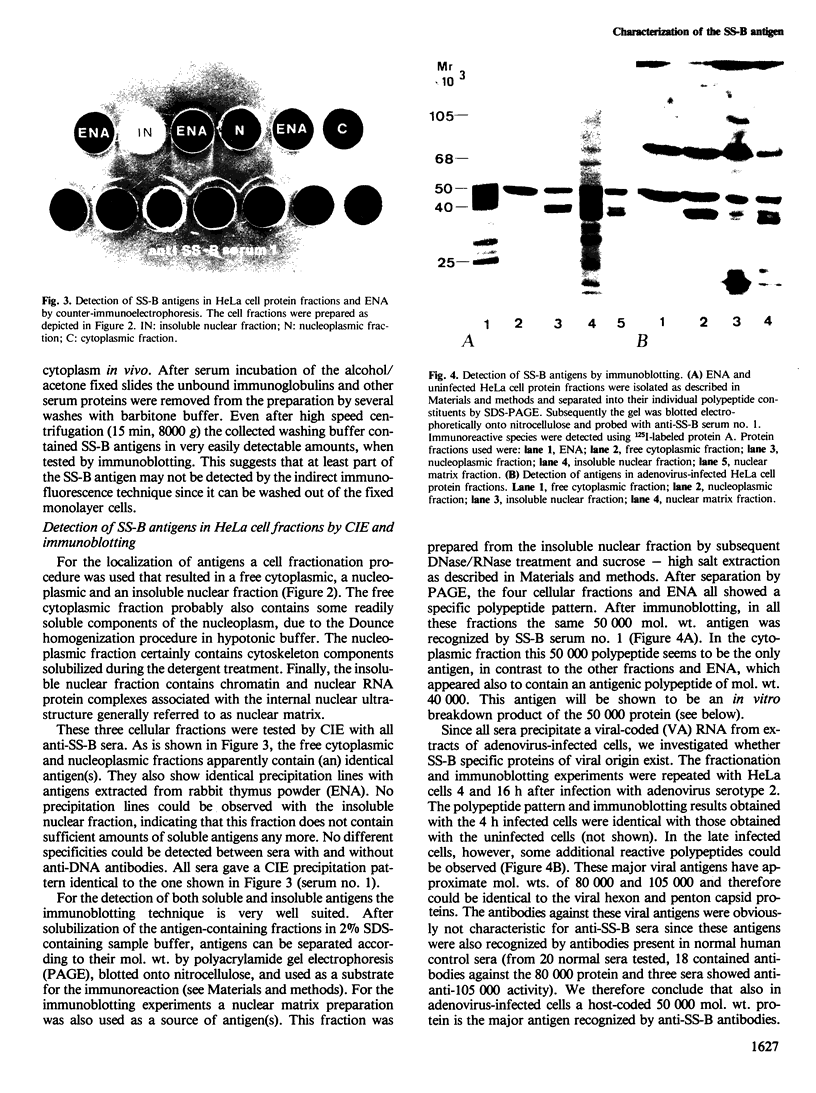
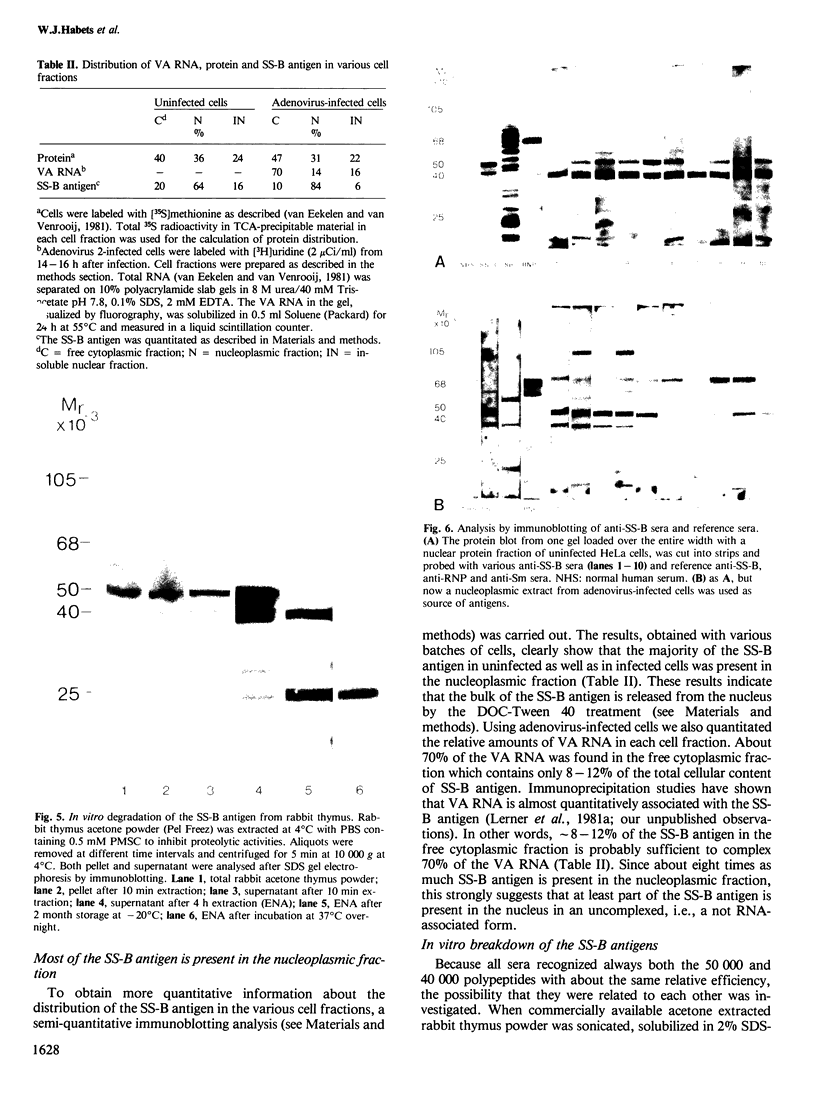
The molecular composition and subcellular localization of the antigens recognized by anti-SS-B (La or Ha) antibodies was investigated. Ten anti-SS-B sera were selected by indirect immunofluorescence and by their immunological identity in counter-immunoelectrophoresis (CIE) with an anti-SS-B reference serum. All sera precipitated virus-associated (VA) RNA from cellular extracts of adenovirus-infected HeLa cells. Earlier results had shown that in adenovirus-infected HeLa cells a cellular 50 000 mol. wt. protein was tightly associated with VA RNA in situ. Our present results indicate that this 50 000 protein is the only SS-B antigen present in adenovirus-infected as well as in uninfected cells. A major part (greater than 80%) of the SS-B antigen is present in a readily extractable, soluble form. The rest is found in an insoluble form tightly associated with an internal nuclear structure that is mostly referred to as the nuclear matrix. Both forms are very susceptible to proteolytic degradation resulting in at least two distinct breakdown products of mol. wts. 40 000 and 25 000. The cellular 50 000 polypeptide is present in extracts of various types of cells and tissues, indicating that this antigen is very well conserved during evolution. The association of the 50 000 mol. wt. antigen with host- as well as viral-coded RNA polymerase III products also suggests an important function for this protein in the metabolism of these small RNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., de Groot E. R., Feltkamp T. E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975 Jun 30;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- Akizuki M., Powers R., Holman H. R. A soluble acidic protein of the cell nucleus which reacts with serum from patients with systemic lupus erythermatosus and Sjögren's syndrome. J Clin Invest. 1977 Feb;59(2):264–272. doi: 10.1172/JCI108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J., Bey E. M., Geddes E. W., Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976 Dec 18;50(54):2124–2128. [PubMed] [Google Scholar]
- Asselbergs F. A., Mathews M. B., Smart J. E. Structural characterization of the proteins encoded by adenovirus early region 2A. J Mol Biol. 1983 Jan 15;163(2):177–207. doi: 10.1016/0022-2836(83)90003-7. [DOI] [PubMed] [Google Scholar]
- Ciejek E. M., Nordstrom J. L., Tsai M. J., O'Malley B. W. Ribonucleic acid precursors are associated with the chick oviduct nuclear matrix. Biochemistry. 1982 Sep 28;21(20):4945–4953. doi: 10.1021/bi00263a018. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata N., Tan E. M. Identification of antibodies to nuclear acidic antigens by counterimmunoelectrophoresis. Arthritis Rheum. 1976 May-Jun;19(3):574–580. doi: 10.1002/art.1780190309. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Matter L., Schopfer K., Wilhelm J. A., Nyffenegger T., Parisot R. F., De Robertis E. M. Molecular characterization of ribonucleoprotein antigens bound by antinuclear antibodies. A diagnostic evaluation. Arthritis Rheum. 1982 Nov;25(11):1278–1283. doi: 10.1002/art.1780251102. [DOI] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C., Goutner A., Choquet C., Venuat A. M., Kayibanda B., Pico J. L., Greaves M. F. Phenotypic characterisation of a unique non-T, non-B acute lymphoblastic leukaemia cell line. Nature. 1977 Jun 30;267(5614):841–843. doi: 10.1038/267841a0. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Pettersson U., Vennström B., Philipson L., Mathews M. B. A new species of virus-coded low molecular weight RNA from cells infected with adenovirus type 2. Cell. 1976 Apr;7(4):585–593. doi: 10.1016/0092-8674(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Takano M., Agris P. F., Sharp G. C. Purification and biochemical characterization of nuclear ribonucleoprotein antigen using purified antibody from serum of a patient with mixed connective tissue disease. J Clin Invest. 1980 Jun;65(6):1449–1456. doi: 10.1172/JCI109809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Van Eekelen C. A., Mariman E. C., Reinders R. J., Van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with host cell proteins. Eur J Biochem. 1981 Oct;119(3):461–467. doi: 10.1111/j.1432-1033.1981.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Venables P. J., Charles P. J., Buchanan R. R., Yi T., Mumford P. A., Schrieber L., Room G. R., Maini R. N. Quantitation and detection of isotypes of anti-SS-B antibodies by ELISA and Farr assays using affinity purified antigens. An approach to the investigation of Sjögren's syndrome and systemic lupus erythematosus. Arthritis Rheum. 1983 Feb;26(2):146–155. doi: 10.1002/art.1780260205. [DOI] [PubMed] [Google Scholar]
- White P. J., Gardner W. D., Hoch S. O. Identification of the immunogenically active components of the Sm and RNP antigens. Proc Natl Acad Sci U S A. 1981 Jan;78(1):626–630. doi: 10.1073/pnas.78.1.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., Salden M. H., Habets W. J., van de Putte L. B., van Venrooij W. J. On the existence of an internal nuclear protein structure in HeLa cells. Exp Cell Res. 1982 Sep;141(1):181–190. doi: 10.1016/0014-4827(82)90080-5. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C., Buijtels H., Linné T., Ohlsson R., Philipson L., van Venrooij W. Detection of a cellular polypeptide associated with adenovirus-coded VA RNA using in vitro labeling of proteins cross-linked to RNA. Nucleic Acids Res. 1982 May 25;10(10):3039–3052. doi: 10.1093/nar/10.10.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C., Ohlsson R., Philipson L., Mariman E., van Beek R., van Venrooij W. Sequence dependent interaction of hnRNP proteins with late adenoviral transcripts. Nucleic Acids Res. 1982 Nov 25;10(22):7115–7131. doi: 10.1093/nar/10.22.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]