Abstract
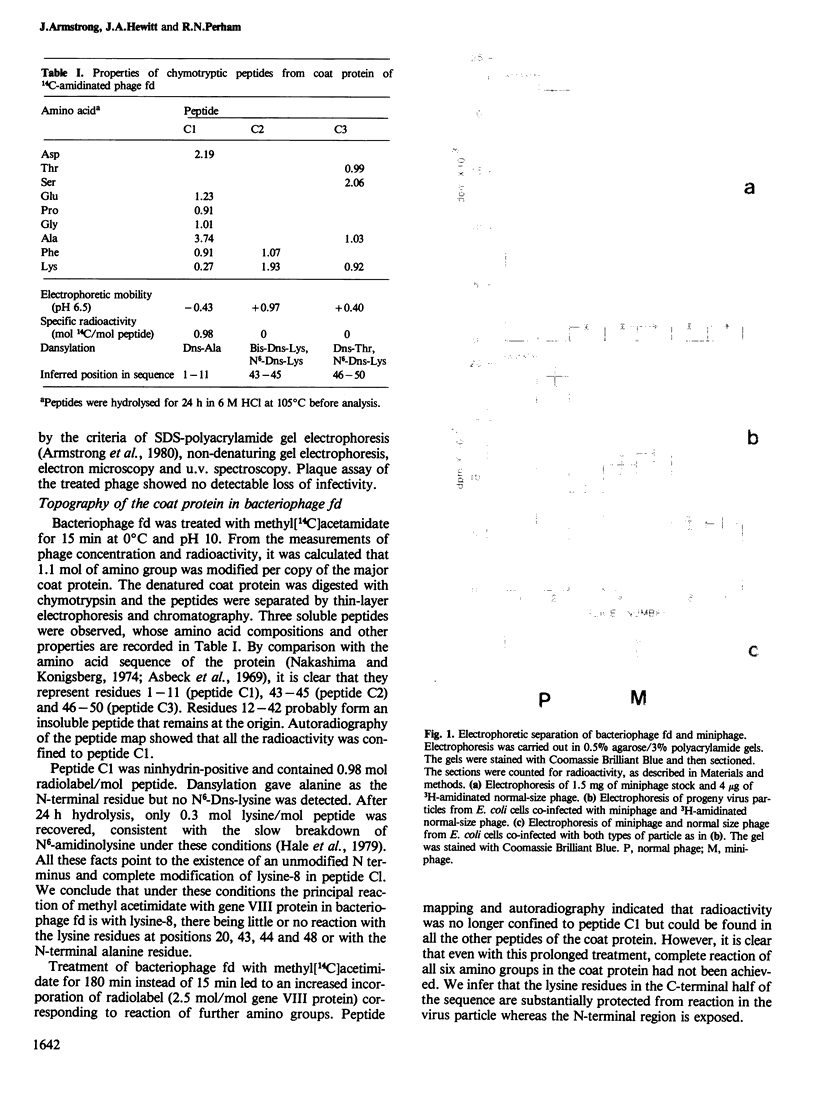
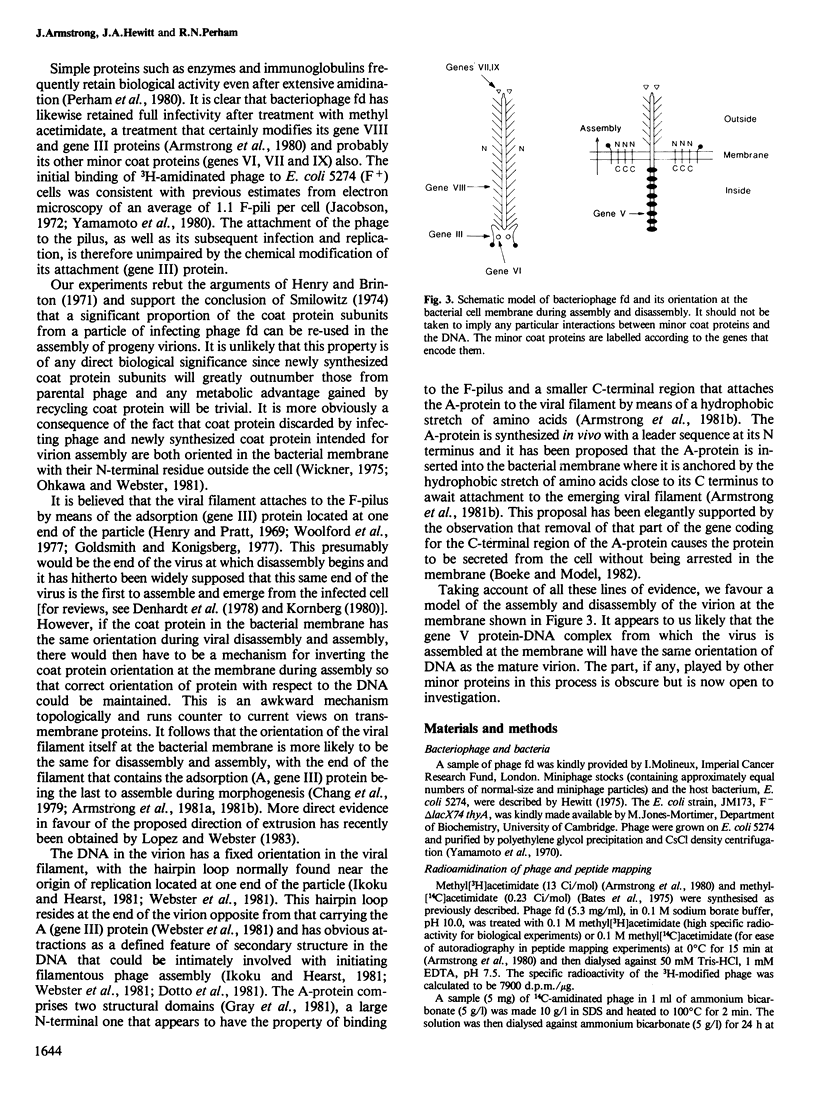
The major (gene VIII) coat protein of bacteriophage fd was radiolabelled by treating the virus with methyl[3H]acetimidate without causing any loss of infectivity. Complete amidination of lysine-8 in the amino acid sequence of the protein was achieved but little or no modification of the lysine residues near the C terminus was observed. This supports the assumption that the coat protein is oriented in the viral filament with its N terminus on the outside and its C-terminal region abutting the DNA. Escherichia coli was co-infected with radiolabelled bacteriophage and with unlabelled miniphage, a shorter defective form of phage fd. Radiolabel was detected in the progeny miniphage, proving that individual coat protein subunits can be recycled and assembled onto progeny miniphage DNA. About 35% of the coat protein subunits of phage particles infecting E. coli were recycled in 1 h. These facts support a model of the assembly and disassembly of the virion at the bacterial membrane in which the end of the particle containing the minor adsorption (gene III) protein, which is presumably the first to disassemble during infection, is the last to assemble during morphogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Leadlay P. F., Perham R. N. Synthesis of methyl[3H]acetimidate of high specific radioactivity, a reagent for radiolabeling proteins. Anal Biochem. 1980 Dec;109(2):410–413. doi: 10.1016/0003-2697(80)90669-7. [DOI] [PubMed] [Google Scholar]
- Armstrong J., Perham R. N., Walker J. E. Domain structure of bacteriophage fd adsorption protein. FEBS Lett. 1981 Nov 30;135(1):167–172. doi: 10.1016/0014-5793(81)80969-6. [DOI] [PubMed] [Google Scholar]
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Banner D. W., Nave C., Marvin D. A. Structure of the protein and DNA in fd filamentous bacterial virus. Nature. 1981 Feb 26;289(5800):814–816. doi: 10.1038/289814a0. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Zink B. Nucleotide sequence and genome organisation of filamentous bacteriophages fl and fd. Gene. 1981 Dec;16(1-3):35–58. doi: 10.1016/0378-1119(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Model P. A prokaryotic membrane anchor sequence: carboxyl terminus of bacteriophage f1 gene III protein retains it in the membrane. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5200–5204. doi: 10.1073/pnas.79.17.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Makowski L. The symmetries of filamentous phage particles. J Mol Biol. 1981 Jan 25;145(3):611–617. doi: 10.1016/0022-2836(81)90549-0. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Model P., Blobel G. Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1251–1255. doi: 10.1073/pnas.76.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Enea V., Zinder N. D. Functional analysis of bacteriophage f1 intergenic region. Virology. 1981 Oct 30;114(2):463–473. doi: 10.1016/0042-6822(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Enea V., Horiuchi K., Turgeon B. G., Zinder N. D. Physical map of defective interfering particles of bacteriophage f1. J Mol Biol. 1977 Apr 25;111(4):395–414. doi: 10.1016/s0022-2836(77)80061-2. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. E., Konigsberg W. H. Adsorption protein of the bacteriophage fd: isolation, molecular properties, and location in the virus. Biochemistry. 1977 Jun 14;16(12):2686–2694. doi: 10.1021/bi00631a016. [DOI] [PubMed] [Google Scholar]
- Grant R. A., Lin T. C., Konigsberg W., Webster R. E. Structure of the filamentous bacteriophage fl. Location of the A, C, and D minor coat proteins. J Biol Chem. 1981 Jan 10;256(1):539–546. [PubMed] [Google Scholar]
- Gray C. W., Brown R. S., Marvin D. A. Adsorption complex of filamentous fd virus. J Mol Biol. 1981 Mar 15;146(4):621–627. doi: 10.1016/0022-2836(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Gray C. W., Carlson R. D. Neutron scattering data on reconstituted complexes of fd deoxyribonucleic acid and gene 5 protein show that the deoxyribonucleic acid is near the center. Biochemistry. 1982 May 25;21(11):2702–2713. doi: 10.1021/bi00540a020. [DOI] [PubMed] [Google Scholar]
- Griffith J., Kornberg A. Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology. 1974 May;59(1):139–152. doi: 10.1016/0042-6822(74)90211-6. [DOI] [PubMed] [Google Scholar]
- Hale G., Hooper E. A., Perham R. N. Amidination of pyruvate dehydrogenase complex of Escherichia coli under denaturing conditions. Biochem J. 1979 Jan 1;177(1):136–137. doi: 10.1042/bj1770136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Brinton C. C., Jr Removal of the coat protein of bacteriophages M13 or fd from the exterior of the host after infection. Virology. 1971 Dec;46(3):754–763. doi: 10.1016/0042-6822(71)90077-8. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Pratt D. The proteins of bacteriophage M13. Proc Natl Acad Sci U S A. 1969 Mar;62(3):800–807. doi: 10.1073/pnas.62.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. A. Miniphage-a class of satellite phage to M13. J Gen Virol. 1975 Jan;26(1):87–94. doi: 10.1099/0022-1317-26-1-87. [DOI] [PubMed] [Google Scholar]
- Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage f1 DNA. J Virol. 1982 Oct;44(1):32–46. doi: 10.1128/jvi.44.1.32-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoku A. S., Hearst J. E. Identification of a structural hairpin in the filamentous chimeric phage M13Gori1. J Mol Biol. 1981 Sep 15;151(2):245–259. doi: 10.1016/0022-2836(81)90514-3. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983 May;127(1):177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Konigsberg W. Reinvestigation of a region of the fd bacteriophage coat protein sequence. J Mol Biol. 1974 Sep 25;88(3):598–600. doi: 10.1016/0022-2836(74)90410-0. [DOI] [PubMed] [Google Scholar]
- Nave C., Brown R. S., Fowler A. G., Ladner J. E., Marvin D. A., Provencher S. W., Tsugita A., Armstrong J., Perham R. N. Pf1 filamentous bacterial virus. X-ray fibre diffraction analysis of two heavy-atom derivatives. J Mol Biol. 1981 Jul 15;149(4):675–707. doi: 10.1016/0022-2836(81)90353-3. [DOI] [PubMed] [Google Scholar]
- Newman J., Swinney H. L., Day L. A. Hydrodynamic properties and structure of fd virus. J Mol Biol. 1977 Nov 5;116(3):593–603. doi: 10.1016/0022-2836(77)90086-9. [DOI] [PubMed] [Google Scholar]
- Ohkawa I., Webster R. E. The orientation of the major coat protein of bacteriophage f1 in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1981 Oct 10;256(19):9951–9958. [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Simons G. F., Konings R. N., Schoenmakers J. G. Genes VI, VII, and IX of phage M13 code for minor capsid proteins of the virion. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4194–4198. doi: 10.1073/pnas.78.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H. Bacteriophage f1 infection: fate of the parental major coat protein. J Virol. 1974 Jan;13(1):94–99. doi: 10.1128/jvi.13.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkner E., Bonhoeffer F., Gierer A. The fate of the protein component of bacteriophage fd during infection. Biochem Biophys Res Commun. 1967 Sep 27;28(6):932–939. doi: 10.1016/0006-291x(67)90069-1. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Grant R. A., Hamilton L. A. Orientation of the DNA in the filamentous bacteriophage f1. J Mol Biol. 1981 Oct 25;152(2):357–374. doi: 10.1016/0022-2836(81)90247-3. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. doi: 10.1073/pnas.72.12.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Steinman H. M., Webster R. E. Adsorption protein of bacteriophage fl: solubilization in deoxycholate and localization in the fl virion. Biochemistry. 1977 Jun 14;16(12):2694–2700. doi: 10.1021/bi00631a017. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kanegasaki S., Yoshikawa M. Effects of temperature and energy inhibitors on complex formation between Escherichia coli male cells and filamentous phage fd. J Gen Microbiol. 1980 Jul;119(1):87–93. doi: 10.1099/00221287-119-1-87. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]




